Drug Trials Snapshots: Ga-68-DOTATOC
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the Ga-68-DOTATOC Package Insert for complete information.
Ga-68-DOTATOC
UHC – PET Imaging Center
Approval date: August 21, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
Ga-68-DOTATOC is a drug for the detection of specific types of tumors called somatostatin receptor positive neuro-endocrine tumors (NETs).
NETs are rare tumors that develop in certain hormone-producing cells of the body’s neuro-endocrine system.
How is this drug used?
Ga-68-DOTATOC is injected into a vein (intravenous) in preparation for an imaging test (called positron emission tomography scan or PET scan) to help detect the tumor.
What are the benefits of this drug?
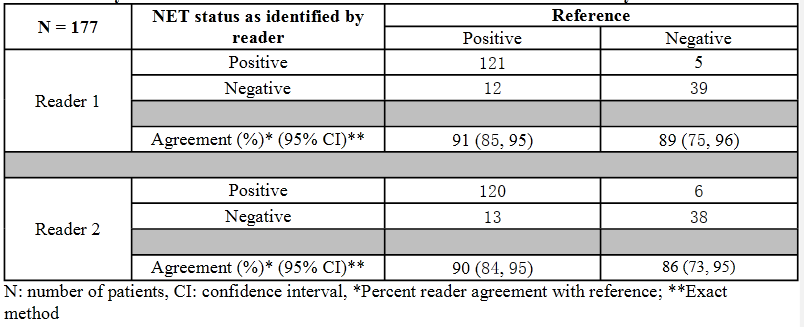
Ga-68-DOTATOC shows the sites of NETs. In Trials 1 and 2, Ga-68-DOTATOC correctly identified NETS about 90%-92% of the time.
What are the benefits of this drug (results of trials used to assess efficacy)?
Efficacy results are summarized below for patients in Trials 1 and 2. The primary outcome was the percent reader agreement with NET diagnosis per composite reference.
Trial 2 included 62 patients with histologically positive NET or other SSTR positive tumor referred for evaluation of Ga-68-DOTATOCdisease before or after treatment. In 59 of the 62 patients, sufficient data to establish NET status per composite reference was available for efficacy evaluation. The estimated positive and negative percent agreements were 92% and 75% for reader 1 and 90% and 75% for reader 2, respectively.
Ga-68-DOTATOC Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: Ga-68-DOTATOC worked similarly in males and females.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in how Ga-68-DOTATOC worked among races could not be determined.
- Age: Ga-68-DOTATOC worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize efficacy results by sex, race, and age group.
Table 3. Subgroup Analysis by Sex
| Positive by Composite Reference | Negative by Composite Reference | ||
|---|---|---|---|
| Trial 1, N=177 | |||
| Female n=103 |
Positive Identified by Consensus Reading* | 61 | 1 |
| Negative Identified by Consensus Reading* | 6 | 35 | |
| Agreement (%) | 91.0 | 97.2 | |
| Male n=74 |
Positive Identified by Consensus Reading* | 61 | 2 |
| Negative Identified by Consensus Reading* | 5 | 6 | |
| Agreement (%) | 92.4 | 75.0 | |
| Trial 2, N=59 | |||
| Female n=30 |
Positive Identified by Consensus Reading* | 24 | 0 |
| Negative Identified by Consensus Reading* | 3 | 3 | |
| Agreement (%) | 88.9 | 100.0 | |
| Male n=29 |
Positive Identified by Consensus Reading* | 23 | 1 |
| Negative Identified by Consensus Reading* | 1 | 4 | |
| Agreement (%) | 95.8 | 80.0 | |
*Two nuclear medicine physicians re-reviewed the images together to determine consensus in a blinded fashion. When they were unable to reach consensus, a third nuclear medicine physician read the images in a blinded fashion to determine the final consensus reading. When the third physician was not able to determine the final consensus reading, the reads were classified as indeterminate.
FDA Review
Table 4. Subgroup Analysis by Race
| Positive by Composite Reference | Negative by Composite Reference | ||
|---|---|---|---|
| Trial 1, N=177a | |||
| White n=168 |
Positive Identified by Consensus Reading* | 118 | 3 |
| Negative Identified by Consensus Reading* | 10 | 37 | |
| Agreement (%) | 92.2 | 92.5 | |
| All Others n=8 |
Positive Identified by Consensus Reading* | 4 | 0 |
| Negative Identified by Consensus Reading* | 1 | 3 | |
| Agreement (%) | 80.0 | 100.0 | |
| Trial 2, N=59 | |||
| White n=57 |
Positive Identified by Consensus Reading* | 45 | 1 |
| Negative Identified by Consensus Reading* | 4 | 7 | |
| Agreement (%) | 91.8 | 87.5 | |
| All Others n=2 |
Positive Identified by Consensus Reading* | 2 | 0 |
| Negative Identified by Consensus Reading* | 0 | 0 | |
| Agreement (%) | 100.0 | – | |
a One patient has an unknown race and is not included in the analysis
* Two nuclear medicine physicians re-reviewed the images together to determine consensus in a blinded fashion. When they were unable to reach consensus, a third nuclear medicine physician read the images in a blinded fashion to determine the final consensus reading. When the third physician was not able to determine the final consensus reading, the reads were classified as indeterminate.
FDA Review
Table 5. Subgroup Analysis by Age Group
| Positive by Composite Reference | Negative by Composite Reference | |||
|---|---|---|---|---|
| Trial 1, N=177 | ||||
| Pediatric (<18 yr.) n=6 |
Positive Identified by Consensus Reading* | 2 | 0 | |
| Negative Identified by Consensus Reading* | 1 | 3 | ||
| Agreement (%) | 66.7 | 100.0 | ||
| Adult (18 to <65 yr.) n=135 |
Positive Identified by Consensus Reading* | 90 | 3 | |
| Negative Identified by Consensus Reading* | 9 | 33 | ||
| Agreement (%) | 90.9 | 91.7 | ||
| Geriatric (≥65 yr.) n=36 |
Positive Identified by Consensus Reading* | 30 | 0 | |
| Negative Identified by Consensus Reading* | 1 | 5 | ||
| Agreement (%) | 96.8 | 100.0 | ||
| Trial 2, N=59 | ||||
| Pediatric (<18 yr.) n=4 |
Positive Identified by Consensus Reading* | 2 | 0 | |
| Negative Identified by Consensus Reading* | 1 | 1 | ||
| Agreement (%) | 66.7 | 100.0 | ||
| Adult (18 to <65 yr.) n=40 |
Positive Identified by Consensus Reading* | 31 | 1 | |
| Negative Identified by Consensus Reading* | 2 | 6 | ||
| Agreement (%) | 93.9 | 85.7 | ||
| Geriatric (≥65 yr.) n=15 |
Positive Identified by Consensus Reading* | 14 | 0 | |
| Negative Identified by Consensus Reading* | 1 | 0 | ||
| Agreement (%) | 93.3 | – | ||
* Two nuclear medicine physicians re-reviewed the images together to determine consensus in a blinded fashion. When they were unable to reach consensus, a third nuclear medicine physician read the images in a blinded fashion to determine the final consensus reading. When the third physician was not able to determine the final consensus reading, the reads were classified as indeterminate.
FDA Review
What are the possible side effects?
Ga-68-DOTATOC is a radioactive drug which may increase the risk of lifetime radiation exposure.
The most common adverse reactions were nausea, itching, and flushing.
What are the possible side effects (results of trials used to assess safety)?
Adverse reactions in the safety population are summarized below.
The safety of Ga-68 DOTATOC injection was evaluated in 334 patients in clinical trials of patients receiving a single dose of Ga-68 DOTATOC injection for imaging known or suspected NET.
The following adverse reactions occurred at a rate of < 2%:
Gastrointestinal Disorders: nausea
The following adverse reactions occurred at a rate of a < 1%:
Skin and Subcutaneous Tissue Disorders: pruritus
Vascular Disorders: flushing
Ga-68-DOTATOC Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar among males and females.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients 18 to 64 years of age and those greater than 65 years of age. The number of patients less than 18 years of age was limited; therefore, differences in the occurrence of side effects among patients less than 18 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize adverse events by sex and age group.
Table 6. Summary of Adverse Events by Sex
| Adverse Events | Male n=144 |
Female n=190 |
|---|---|---|
| Number of Adverse Events | 31 | 44 |
| Patients with Adverse Events, n (%) | 22 (15.3) | 30 (15.8) |
| Adverse Events by Grade | ||
| Grade 1 | 31 (100.0) | 41 (93.2) |
| Grade 2 | 0 | 2 (4.5) |
| Grade 3 | 0 | 1 (2.3) |
FDA Review
Table 7. Summary of Adverse Events by Age
| Adverse Events | < 18 years n=18 |
18 to 65 years n=256 |
>65 years n=60 |
|---|---|---|---|
| Number of Adverse Events | 2 | 61 | 12 |
| Patients with Adverse Events, n (%) | 2 (18.2) | 41 (16.0) | 7 (11.7) |
| Adverse Events by Grade | |||
| Grade 1 | 2 (100.0) | 58 (95.1) | 12 (100.0) |
| Grade 2 | 0 | 2 (3.3) | 0 |
| Grade 3 | 0 | 1 (1.6) | 0 |
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved Ga-68-DOTATOC based on evidence from three clinical trials (Trial 1/NCT#1619865, Trial 2/NCT#1869725, Trial 3/NCT#2441062) of 334 known or suspected neuro-endocrine tumors. The trials were conducted in the United States.
Table 9 summarizes demographics for the patients used to evaluate the benefits of Ga-68-DOTATOC in trials 1 and 2 (efficacy population).
The figure below summarizes how many males and females were used to evaluate safety in trials 1, 2, and 3 (safety population).
Figure 1. Baseline Demographics by Sex (safety population)
FDA Review
Figure 2 and Table 1 summarize the percentage of patients by race used to evaluate safety in the clinical trials.
Figure 2. Baseline Demographics by Race (safety population)
FDA Review
Table 1. Demographics of Trials by Race (safety population)
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 314 | 94% |
| Black or African American | 5 | 2% |
| Asian | 4 | 1% |
| American Indian or Alaska Native | 1 | Less than 1% |
| Other | 7 | 2% |
| Missing | 3 | 1% |
FDA Review
Figure 3 summarizes the percentage of patients by age group in the clinical trials used to evaluate safety.
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The tables below summarize baseline demographic information for the safety and efficacy populations.
Table 8. Demographic Characteristics (safety population)
| Demographic Parameter | N=334 n (%) |
|---|---|
| Sex | |
| Male | 144 (43.1) |
| Female | 190 (56.9) |
| Race | |
| White | 314 (94.0) |
| Black or African American | 5 (1.5) |
| Asian | 4 (1.2) |
| American Indian or Alaska Native | 1 (0.3) |
| Other | 7 (2.1) |
| Missing | 3 (0.9) |
| Age Group (years) | |
| < 2 | 1 (0.3) |
| 2 to < 12 | 6 (1.8) |
| 12 to < 18 | 11 (3.3) |
|
256 (76.7) |
| > 65 | 60 (18.0) |
| Ethnicity | |
| Hispanic | 7 (2.1) |
| Non-Hispanic | 324 (97.0) |
| Missing | 3 (0.9) |
| Region | |
| United States | 334 (100.0) |
FDA Review
Table 9. Demographic Characteristics (efficacy population)
| Demographic Parameter | Trial 1 N=220 n (%) |
Trial 2 N=62 n (%) |
|---|---|---|
| Sex | ||
| Female | 134 (60.9) | 31 (50.0) |
| Male | 86 (39.1) | 31 (50.0) |
| Race | ||
| White | 207 (94.1) | 60 (96.8) |
| Black or African American | 2 (0.9) | 1 (1.6) |
| Asian | 2 (0.9) | 1 (1.6) |
| American Indian/Alaskan Native | 1 (0.5) | 0 |
| Other | 5 (2.3) | 0 |
| Missing | 3 (1.4) | 0 |
| Age Group | ||
| <18 years | 8 (3.6) | 5 (8.1) |
| 18 to <65 years | 174 (79.1) | 41 (66.1) |
| ≥65 years | 38 (17.3) | 16 (25.8) |
| Ethnicity, n (%) | ||
| Hispanic | 5 (2.3) | 1 (1.6) |
| Not Hispanic | 213 (96.8) | 60 (96.8) |
| Unknown | 2 (0.9) | 1 (1.6) |
| Region | ||
| United States | 220 (100.0) | 62 (100.0) |
FDA Review
How were the trials designed?
The benefit and safety of Ga-68-DOTATOC in detecting neuro-endocrine tumors was evaluated in three clinical trials. Patients had known or suspected neuroendocrine tumors. All patients received a single intravenous dose of Ga-68 DOTATOC. Images were read by two readers. In all trials, the image readers did not know whether the patients had neuroendocrine tumors.
In Trials 1 and 2, the benefit of Ga-68-DOTATOC was assessed by comparing the accuracy of Ga-68-DOTATOC images to detect neuroendocrine tumors in patients whose NETs status was known using a tumor biopsy or other imaging techniques.
Patients in Trial 3 were primarily evaluated for side effects.
How were the trials designed?
The safety and efficacy of Ga-68-DOTATOC were established in 3 open-label, single-arm trials that enrolled patients from 4 to 82 years of age with known or suspected SSTR-positive NETs. Patients received a single dose of Ga-68 DOTATOC. The imaging results were compared to a composite reference consisting of histopathology and imaging (MR, CT, or In-111 pentetreotide imaging) acquired within 1 year of the Ga-68 DOTATOC imaging, as well as chromogranin A and pancreastatin levels.
The primary outcome of Trials 1 and 2 was the percent agreement of imaging results to composite reference imaging. The proportion of patients positive for NET per composite reference who were identified as positive by the Ga-68 DOTATOC image was used to quantify positive percent agreement. The proportion of patients without NET per composite reference who were identified as negative by the Ga-68 DOTATOC image was used to quantify negative percent agreement.
The FDA used Trial 3 to primarily evaluate adverse events.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.