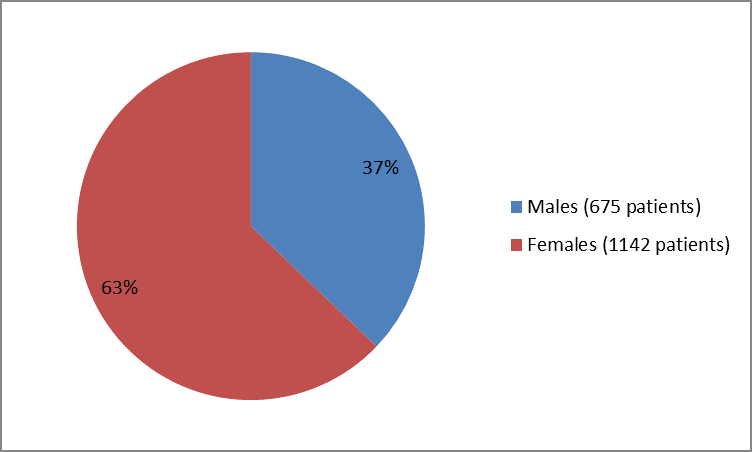Drug Trials Snapshots: FASENRA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to FASENRA Prescribing Information for complete information.
FASENRA (benralizumab)
fas-en-rah
AstraZeneca
Approval date: November 14, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
FASENRA is a drug for the treatment of specific type of severe asthma (called eosinophilic phenotype asthma) in patients 12 years and older whose asthma is not well controlled with current medications. FASENRA is to be used in addition to asthma maintenance medications.
How is this drug used?
FASENRA is given by a healthcare provider using a needle placed under the skin one time every 4 weeks for the first 3 doses, and then every 8 weeks.
What are the benefits of this drug?
Patients who received FASENRA had fewer asthma attacks that required a stay in the hospital and/or a visit to the emergency room, and had greater reduction in their daily maintenance dose of oral corticosteroids.
What are the benefits of this drug (results of trials used to assess efficacy)?
The tables below summarize efficacy results for the clinical trials. The primary endpoint for Trials 1 and 2 was the rate of asthma exacerbations defined as a worsening of asthma requiring use of oral/systemic corticosteroids for at least 3 days, and/or emergency department visits requiring use of oral/systemic corticosteroids and/or hospitalization.
Table 2. Rate of Exacerbations, Trial 1 and 2 (ITT Population) a
| Trial | Treatment | Exacerbations per year | ||
|---|---|---|---|---|
| Rate | Difference | Rate Ratio (95% CI) | ||
| All exacerbations | ||||
| Trial 1 | FASENRA b (n=267) | 0.74 | -0.78 | 0.49 (0.37, 0.64) |
| Placebo (n=267) | 1.52 | -- | -- | |
| Trial 2 | FASENRA b (n=239) | 0.73 | -0.29 | 0.72 (0.54, 0.95) |
| Placebo (n=248) | 1.01 | -- | -- | |
| Exacerbations requiring hospitalization/emergency room visit | ||||
| Trial 1 | FASENRA b (n=267) | 0.09 | -0.16 | 0.37 (0.20, 0.67) |
| Placebo (n=267) | 0.25 | -- | -- | |
| Trial 2 | FASENRA b (n=239) | 0.12 | 0.02 | 1.23 (0.64, 2.35) |
| Placebo (n=248) | 0.10 | -- | -- | |
| Exacerbations requiring hospitalization | ||||
| Trial 1 | FASENRA b (n=267) | 0.07 | -0.07 | 0.48 (0.22, 1.03) |
| Placebo (n=267) | 0.14 | -- | -- | |
| Trial 2 | FASENRA b (n=239) | 0.07 | 0.02 | 1.48 (0.65, 3.37) |
| Placebo (n=248) | 0.05 | -- | -- | |
aRandomized patients
bFASENRA 30mg administered every 4 weeks for the first 3 doses, and every 8 weeks thereafter
FASENRA Prescribing Information
Oral Corticosteroid (OCS) Reduction, Trial 3
The primary endpoint in Trial 3 was percent reduction from baseline of the final OCS dose during Weeks 24 to 28, while maintaining asthma control.
The median percent reduction in daily OCS dose from baseline was 75% in patients receiving FASENRA (95% CI: 60, 88) compared to 25% in patients receiving placebo (95% CI: 0, 33).
FASENRA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: FASENRA worked similarly in men and women.
- Race: FASENRA worked similarly among races studied.
- Age: FASENRA worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The figures below summarize efficacy results by subgroups for Trials 1 and 2 separately.
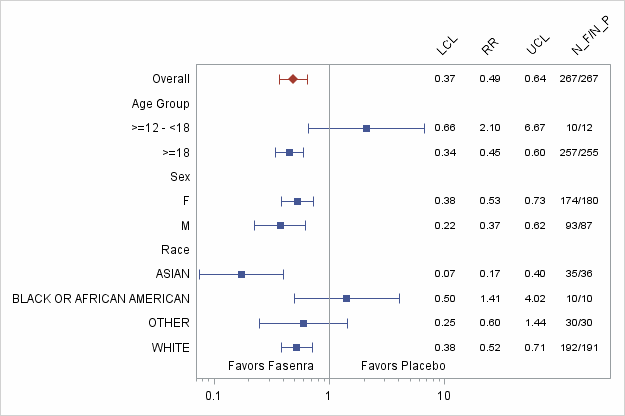
Figure 4. Annual Asthma Exacerbation Rate Ratio for FASENRA vs. Placebo by Subgroup (FAS) -Trial 1
Abbreviations: RR: Estimated rate ratio of FASENRA Q8W vs. Placebo. LCL: Lower confidence limit of the 95% confidence interval for rate ratio; UCL: Upper confidence limit of the 95% confidence interval for rate ratio; N_F: Number of FASENRA patients Q8W dosing; N_P: Number of patients treated with Placebo.
Adapted from FDA Statistical review
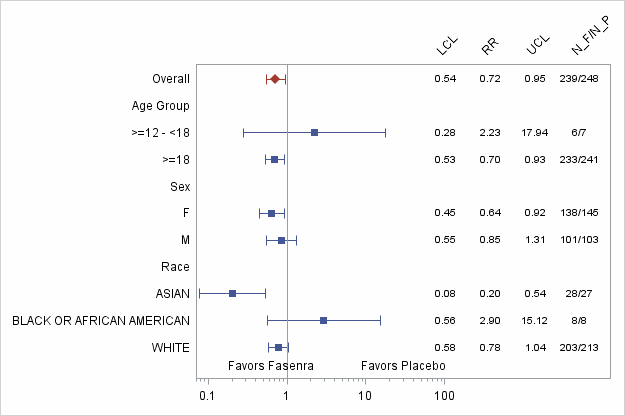
Figure 5. Annual Asthma Exacerbation Rate for FASENRA vs. Placebo by Subgroup (FAS)-Trial 2
Abbreviations: RR: Estimated rate ratio of FASENRA Q8W vs. Placebo. LCL: Lower confidence limit of the 95% confidence interval for rate ratio; UCL: Upper confidence limit of the 95% confidence interval for rate ratio; N_F: Number of FASENRA patients Q8W dosing; N_P: Number of patients treated with Placebo.
Adapted from FDA Statistical review
What are the possible side effects?
FASENRA may cause severe allergic reactions including a life threatening allergic reaction called anaphylaxis.
The most common side effect of FASENRA are headache and sore throat.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in at least 3% of patients and more frequently for FASENRA in pooled Trials 1 and 2 in patients with severe asthma.
Table 4. Adverse Reactions with FASENRA with Greater than or Equal to 3% Incidence in Patients with Asthma (Trials 1 and 2)
| Adverse Reactions | FASENRA (N= 822) % | Placebo (N=847) % |
|---|---|---|
| Headache | 8 | 6 |
| Pyrexia | 3 | 2 |
| Pharyngitis* | 5 | 3 |
| Hypersensitivity reactions** | 3 | 3 |
* Pharyngitis was defined by the following terms: ‘Pharyngitis’, ‘Pharyngitis bacterial’, ‘Viral pharyngitis’, ‘Pharyngitis streptococcal’.
** Hypersensitivity Reactions were defined by the following terms: ‘Urticaria’, ‘Urticaria papular’, and ‘Rash’
Adverse reactions from Trial 3 with 28 weeks of treatment with FASENRA (n = 73) or placebo (n = 75) in which the incidence was more common in FASENRA than placebo include headache (8% compared to 5, respectively) and pyrexia (3% compared to 1%, respectively).
FASENRA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar among races studied.
- Age: The occurrence of side effects was similar in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Subgroup analyses of treatment emergent adverse events (TEAEs) in safety population of pooled Trials 1 and 2 is presented below. Trial 3 data are presented separately.
Table 5. Adverse Events by Subgroups-Pooled Trials 1 and 2 (safety population)
| Subgroup | FASENRA N=822 | Placebo N=847 | Relative Risk (95% CI) | ||
|---|---|---|---|---|---|
| n(%) | Total,N | n(%) | Total,N | ||
| Overall TEAEs | 605 (73.6) | 822 | 661 (78.0) | 847 | 0.94 (0.89, 1.00) |
| Sex | |||||
| Men | 210 (68.2) | 308 | 228 (72.6) | 314 | 0.94 (0.85, 1.04) |
| Women | 395 (76.8) | 514 | 433 (81.2) | 533 | 0.95 (0.89, 1.01) |
| Age (years) | |||||
| >= 12 > | 25 (65.8) | 38 | 30 (65.2) | 46 | 1.01 (0.74, 1.38) |
| >= 18 > | 256 (72.9) | 351 | 273 (79.6) | 343 | 0.92 (0.84, 1.00) |
| >= 50 > | 249 (72.8) | 342 | 257 (76.0) | 338 | 0.96 (0.88, 1.05) |
| >= 65 > | 75 (82.4) | 91 | 101 (84.2) | 120 | 0.98 (0.87, 1.11) |
| Race | |||||
| White | 471 (73.7) | 639 | 519 (77.0) | 674 | 0.96 (0.90, 1.02) |
| Black or African American | 22 (73.3) | 30 | 26 (86.7) | 30 | 0.85 (0.65, 1.09) |
| Asian | 82 (78.1) | 105 | 85 (82.5) | 103 | 0.95 (0.83, 1.08) |
| American Indian or Alaskan Native | 6 (60.0) | 10 | 11 (91.7) | 12 | 0.65 (0.38, 1.12) |
| Native Hawaiian or Pacific Islander | 0 (0.0) | 0 | 2 (100.0) | 2 | -- |
| Other | 24 (63.2) | 38 | 18 (69.2) | 26 | 0.91 (0.64, 1.30) |
| Ethnicity | |||||
| Hispanic | 113 (62.8) | 180 | 127 (75.1) | 169 | 0.84 (0.72, 0.96) |
| Non-Hispanic | 492 (76.6) | 642 | 534 (78.8) | 678 | 0.97 (0.92, 1.03) |
| Region | |||||
| United States | 95 (79.2) | 120 | 113 (87.6) | 129 | 0.90 (0.81, 1.01) |
| Rest of the World | 510 (72.6) | 702 | 548 (76.3) | 718 | 0.95 (0.90, 1.01) |
Adapted from FDA Clinical review
Table 6. Adverse Events by Subgroups- Trial 3 (safety population)
| Subgroup | FASENRA N=73 | Placebo N=75 | Risk Difference (95% CI) | ||
|---|---|---|---|---|---|
| n(%) | Total,N | n(%) | Total,N | ||
| Overall TEAEs | 55 (75.3) | 73 | 62 (82.7) | 75 | -7.32 (-20.41, 5.76) |
| Sex | |||||
| Male | 23 (88.5) | 26 | 20 (74.1) | 27 | 14.39 (-6.20, 34.98) |
| Female | 32 (68.1) | 47 | 42 (87.5) | 48 | -19.41 (-35.70, -3.13) |
| Age (years) | |||||
| >= 18 > | 20 (69.0) | 29 | 30 (83.3) | 36 | -14.37 (-35.15, 6.41) |
| >= 50 > | 25 (78.1) | 32 | 26 (83.9) | 31 | -5.75 (-25.05, 13.56) |
| >= 65 > | 10 (83.3) | 12 | 6 (75.0) | 8 | 8.33 (-28.34, 45.01) |
| Race | |||||
| White | 49 (74.2) | 66 | 57 (81.4) | 70 | -7.19 (-21.13, 6.75) |
| Black or African American | 1 (100.0) | 1 | 1 (100.0) | 1 | -- |
| Asian | 4 (80.0) | 5 | 4 (100.0) | 4 | -20.00 (-55.06, 15.06) |
| Other | 1 (100.0) | 1 | 0 (0.0) | 0 | -- |
| Ethnicity | |||||
| Hispanic | 3 (60.0) | 5 | 10 (100.0) | 10 | -40.00 (-82.94, 2.94) |
| Non-Hispanic | 52 (76.5) | 68 | 52 (80.0) | 65 | -3.53 (-17.54, 10.48) |
| Region | |||||
| United States | 4 (100.0) | 4 | 4 (80.0) | 5 | 20.00 (-15.06, 55.06) |
| Rest of the World | 51 (73.9) | 69 | 58 (82.9) | 70 | -8.94 (-22.56, 4.67) |
Adapted from Clinical review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved FASENRA based on the evidence from three clinical trials (Trial 1 [NCT01928771], Trial 2 [NCT01914757], Trial 3 [NCT02075255]) of 1817 patients with severe asthma. The trials were conducted in the USA, Canada, South America, Europe, Asia and Africa.
The figure below summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
FDA review
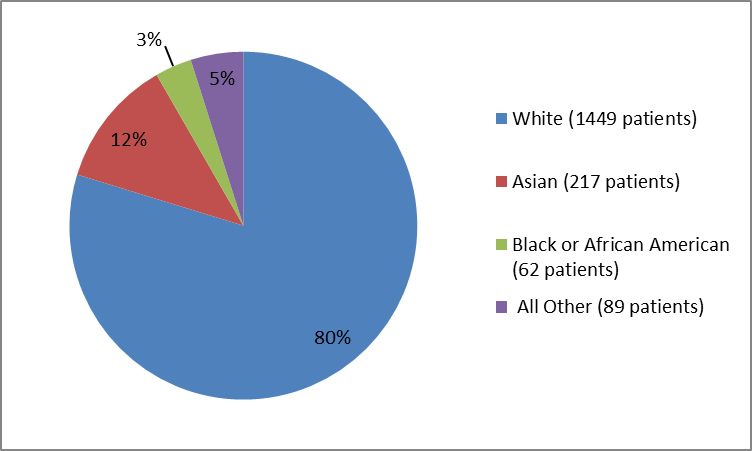
Figure 2 and Table 1 below summarize the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
FDA review
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 1449 | 80 |
| Black or African American | 62 | 3 |
| Asian | 217 | 12 |
| American Indian or Alaskan Native | 22 | 1 |
| Native Hawaiian or Pacific Islander | 2 | less than 1 |
| Other | 65 | 4 |
FDA review
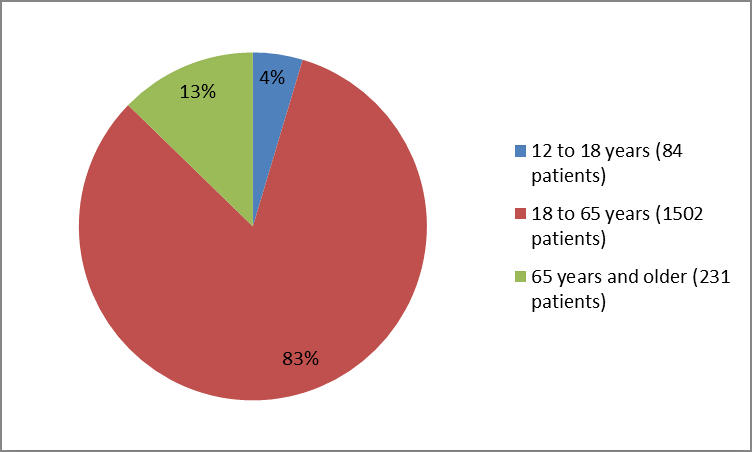
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age
FDA review
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trials. Presented is safety population for pooled Trials 1 and 2 and separately for Trial 3.
Table 7. Baseline Demographics of Patients in the Clinical Trials 1 and 2 (safety population)
| FASENRA N = 822 | Placebo N = 847 | Total N = 1669 | |
|---|---|---|---|
| Age , n (%) | |||
| ≥ 12 to > | 38 (5) | 46 (5) | 84 (5) |
| ≥ 18 to > | 351 (43) | 343 (41) | 694 (42) |
| ≥ 50 to > | 342 (42) | 338 (40) | 680 (41) |
| ≥ 65 | 91 (11) | 120 (14) | 211 (13) |
| Sex, n % | |||
| Male | 308 (38) | 314 (37) | 622 (37) |
| Female | 514 (63) | 533 (63) | 1047 (63) |
| Race, n % | |||
| White | 639 (78) | 674 (80) | 1313 (79) |
| Black or African American | 30 (4) | 30 (4) | 60 (4) |
| Asian | 105 (13) | 103 (12) | 208 (13) |
| America Indian or Alaskan Native | 10 (1) | 12 (1) | 22 (1) |
| Native Hawaiian or Pacific Islander | 0 | 2 (> | 2 (> |
| Other | 38 (5) | 26 (3) | 64 (4) |
| Ethnicity | |||
| Hispanic or Latino | 180 (22) | 169 (20) | 349 (21) |
| Not Hispanic or Latino | 642 (78) | 678 (80) | 1320 (79) |
| Region | |||
| United States | 120 (15) | 129 (15) | 249 (15) |
| Rest of World | 702 (85) | 718 (85) | 1420 (85) |
| Canada | 19 (2) | 20 (2) | 39 (2) |
| South America | 148 (18) | 147 (17) | 295 (18) |
| Europe | 404 (49) | 422 (50) | 826 (49) |
| Asia | 108 (13) | 106 (13) | 214 (13) |
| Africa | 8 (1) | 8 (1) | 16 91) |
| Other | 15 (2) | 15 (2) | 30 (20 |
Adapted from FDA Clinical review
Table 8. Baseline Demographics of Patients in the Clinical Trial 3 (safety population)
| FASENRA N=73 | Placebo N=75 | Total N=148 | |
|---|---|---|---|
| Age group, n (%) | |||
| ≥ 18 to > | 29 (40) | 36 (48) | 65 (44) |
| ≥ 50 to > | 32 (44) | 31 (41) | 63 (43) |
| ≥ 65 | 12 (16) | 8 (11) | 20 (13) |
| Sex, n % | |||
| Men | 26 (36) | 27 (36) | 53 (36) |
| Women | 47 (64) | 48 (64) | 95 (64) |
| Race, n % | |||
| White | 66 (90) | 70 (93) | 136 (92) |
| Black or African American | 1(1) | 1 (1) | 2 (1) |
| Asian | 5 (7) | 4 (5) | 9 (6) |
| Other | 1 (1) | 0 | 1 (1) |
| Ethnicity | |||
| Hispanic or Latino | 5 (7) | 10 (13) | 15 (10) |
| Not Hispanic or Latino | 68 (93) | 65 (87) | 133 (90) |
| Region | |||
| Europe | 49 (67) | 51 (68) | 100 (68) |
| North America | 13 (18) | 14 (19) | 27 (18) |
| Asia | 5(7) | 3(4) | 8 (5) |
| Other | 6 (8) | 7(9) | 13 (9) |
Adapted from FDA Statistical review
How were the trials designed?
The benefit and side effects of FASENRA were evaluated in three clinical trials of patients with severe asthma and increased eosinophilic white cells in blood. All patients were taking their usual treatments for asthma. In addition, patients received new treatment with either FASENRA or placebo. Neither the patients nor the health care providers knew which new treatment was being given until after the trials were completed.
In the first two trials the benefit of FASENRA was evaluated by measuring the frequency of asthma attacks (exacerbations) in comparison to placebo, and in the third trial by measuring the use of maintenance corticosteroids in comparison to placebo.
How were the trials designed?
The safety and efficacy of FASENRA were established in three double-blind, multicenter, randomized, parallel-group, placebo-controlled trials, in adolescents and adults with uncontrolled severe asthma. All patients had to have elevated blood eosinophil count and continued their background asthma therapy throughout the duration of the trials. FASENRA or placebo were administered once every 4 weeks for the first 3 doses, and then every 8 weeks thereafter.
The efficacy outcome measure in Trials 1 and 2 was the frequency of asthma exacerbations for each patient during the 48 and 56-weeks, respectively. Asthma exacerbation was defined as a worsening of asthma requiring use of oral/systemic corticosteroids for at least 3 days, and/or emergency department visits requiring use of oral/systemic corticosteroids and/or hospitalization.
The primary outcome for Trial 3 was percent reduction from baseline of the final OCS dose during Weeks 24 to 28, while maintaining asthma control.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.