Drug Trials Snapshots: EPCLUSA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the EPCLUSA Prescribing Information for complete information.
EPCLUSA (sofosbuvir and velpatasvir)
(ep-KLOO-suh)
Gilead Sciences
Approval date: June 28, 2016
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
EPCLUSA is a drug for the treatment of adults who have a specific type of Hepatitis C virus (HCV) infection, called chronic Hepatitis C virus genotypes 1, 2, 3, 4, 5 or 6 infection.
EPCLUSA is a combination of two anti-viral drugs: sofosbuvir (previously approved for HCV treatment) and velpatasvir (new drug for HCV). It is intended to be used as follows:
- EPLCUSA alone in patients who do not have cirrhosis or who have an early stage cirrhosis
- EPCLUSA in combination with another approved HCV drug called ribavirin in patients who have advanced cirrhosis
Hepatitis C is a viral disease that causes inflammation of the liver that can lead to decreased liver function, cirrhosis, liver failure, liver cancer or death.
How is this drug used?
EPCLUSA is a tablet that is taken once a day for 12 weeks, with or without ribavirin.
What are the benefits of this drug?
EPCLUSA may clear the body of the hepatitis C virus as measured by a blood test 12 weeks after finishing treatment.
What are the benefits of this drug?
The tables below (Tables 2, 3 and 4) summarize efficacy results for the three clinical trials in patients with HCV and either no cirrhosis or with compensated cirrhosis. The primary endpoint was Sustained Virologic Response (SVR) measured at 12 weeks after cessation of treatment and was defined as HCV RNA below the lower limit of quantification (SVR12).
Table 2. Trial 1: Virologic Outcomes by HCV Genotype in EPCLUSA-Treated Subjects without Cirrhosis or with Compensated Cirrhosis (12 Weeks after Treatment)
| EPCLUSA 12 Weeks (N=624) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Total (all GTs) (N=624) |
GT-1 | GT-2 (N=104) |
GT-4 (N=116) |
GT-5 (N=35) |
GT-6 (N=41) |
|||
| GT-1a (N=210) |
GT-1b (N=118) |
Total (N=328) |
||||||
| SVR12 | 99% (618/624) | 98% (206/210) | 99% (117/118) | 98% (323/328) | 100% (104/104) | 100% (116/116) | 97% (34/35) | 100% (41/41) |
| Outcome for Subjects without SVR | ||||||||
| On-Treatment Virologic Failure | 0/624 | 0/210 | 0/118 | 0/328 | 0/104 | 0/116 | 0/35 | 0/41 |
| Relapsea | 1%> (2/623) |
1%> (1/209) |
1% (1/118) |
1% (2/327) |
0/104 | 0/116 | 0/35 | 0/41 |
| Otherb | 1% (4/624) |
1% (3/210) |
0/118 | 1% (3/328) |
0/104 | 0/116 | 3% (1/35) |
0/41 |
GT = genotype; no subjects in the placebo group achieved SVR12.
a. The denominator for relapse is the number of subjects with HCV RNA
b. Other includes subjects who did not achieve SVR and did not meet virologic failure criteria.
EPCLUSA Prescribing Information
Table 3. Trial 2: Virologic Outcomes in Subjects with Genotype 2 HCV without Cirrhosis or with Compensated Cirrhosis (12 Weeks after Treatment)
| EPCLUSA 12 Weeks (N=134) |
SOF + RBV 12 Weeks (N=132) |
|
|---|---|---|
| SVR12 | 99% (133/134) | 94% (124/132) |
| Treatment difference +5.2%; 95% confidence interval (+0.2% to +10.3%) |
||
| Outcome for subjects without SVR | ||
| On-Treatment Virologic Failure | 0/134 | 0/132 |
| Relapsea | 0/133 | 5% (6/132) |
| Otherb | 1% (1/134) | 2% (2/132) |
SOF = sofosbuvir; RBV = ribavirin
a. The denominator for relapse is the number of subjects with HCV RNA
b. Other includes subjects who did not achieve SVR12 and did not meet virologic failure criteria.
EPCLUSA Prescribing Information
Table 4. Trial 3: Virologic Outcomes in Subjects with Genotype 3 HCV without Cirrhosis or with Compensated Cirrhosis
| EP CLUSA 12 Weeks ( N=277) |
S O F + RBV 24 Weeks ( N=275) |
|
|---|---|---|
| SVR12 | 95% (264/277) | 80% (221/275) |
| Treatment difference +14.8%; 95% confidence interval (+9.6% to +20.0%) |
||
| Outcome for subjects without SVR | ||
| On-Treatment Virologic Failure | 0/277 | 1%> |
| Relapsea | 4% (11/276) | 14% (38/272) |
| Otherb | 1% (2/277) | 5% (15/275) |
SOF = sofosbuvir; RBV = ribavirin
a. The denominator for relapse is the number of subjects with HCV RNA
b. Other includes subjects who did not achieve SVR and did not meet virologic failure criteria.
EPCLUSA Prescribing Information
The table below (Table 5) summarizes efficacy results for the clinical trial (Trial 4) in patients with HCV and decompensated cirrhosis. The patients were treated with combination of EPCLUSA and ribavirin. The primary endpoint was Sustained Virologic Response (SVR) measured at 12weeks after cessation of treatment and was defined as HCV RNA below the lower limit of quantification (SVR12).
Table 5. Trial 4: Virologic Outcomes in Subjects with Decompensated Cirrhosis After 12 Weeks of Treatment by HCV Genotype*
| EPCLUSA + RBV 12 Weeks (N=87) |
||
|---|---|---|
| SVR12 | Virologic Failure (relapse and on-treatment failure) |
|
| Overall SVR12a | 94% (82/87) | 3% (3/87) |
| Genotype 1 | 96% (65/68) | 1% (1/68)b |
| Genotype 1a | 94% (51/54) | 2% (1/54)b |
| Genotype 1b | 100% (14/14) | 0% (0/14) |
| Genotype 3 | 85% (11/13) | 15% (2/13)c |
*All subjects with genotype 2 (N=4) and genotype 4 (N=2) HCV infection treated with EPCLUSA and ribavirin achieved SVR12. No subjects with genotype 5 or 6 HCV were treated with EPCLUSA with ribavirin for 12 weeks.
RBV = ribavirin
a. Includes subjects with baseline CPT C cirrhosis: all 4 subjects achieved SVR12.
b. This subject with genotype 1a experienced relapse.
c. One subject had on-treatment virologic failure; pharmacokinetic data from this subject was consistent with non- adherence
EPCLUSA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race, and age.
- Sex: EPCLUSA worked similarly in men and women.
- Race: EPCLUSA worked similarly in all races studied.
- Age: EPCLUSA worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Tables below (Tables 6, 7, 8 and 9) present efficacy results by subgroup. Trials are presented separately due to differences in design and populations studied.
Table 6. Subgroup analysis of SVR12 for Trial 1
| EPCLUSA 12 Weeks (N=624) |
95% CI | |
| Age 65="" years=""> ≥ 65 years |
98.9% (530/536) 100% (88/88) |
(97.6%,99.6%) (95.9%, 100%) |
| Sex Men Women |
98.7% (369/374) 99.6% (249/250) |
(96.9%,99.6%) (97.8%, 100%) |
| Race White Black/African American Other |
99.0% (488/493) 98.1 % (51/52) 100% (76/76) |
(97.6%,99.7%) (89.7%,100%) (95.3%, 100%) |
Adapted from FDA Statistical Review
Table 7. Subgroup analysis of SVR12 for Trial 2
| EPCLUSA 12 Weeks (N=134) |
SOF + RBV 12 Weeks (N=132) |
Diff in SVR12 rate (95% CI) |
|
| Age (years) 65=""> ≥ 65 |
99.1% (105/106) 100% (28/28) |
|
6.3% (1.1%, 12.9%) 0.0% (-12.4%, 15.5%) |
| Sex Men Women |
98.8% (85/86) 100% (48/48) |
90.3% (65/72) 98.3% (59/60) |
8.6% (1.4%, 17.8%) 1.7% (-5.8%, 9.2%) |
| Race White Black/African American Other |
100% (124/124) 83.3% (5/6) 100% (2/2) |
94.6% (105/111) 83.3% (10/12) 100% (8/8) |
5.4% (1.7%, 11.4%) 0.0% (-47.6%, 37.4%) 0.0% (-84.2%, 44.0%) |
SOF = sofosbuvir; RBV = ribavirin
Adapted from FDA Statistical Review
Table 8. Subgroup analysis of SVR12 for Trial 3
| EPCLUSA 12 weeks (N=277) |
SOF + RBV 24 weeks (N=275) |
Diff in SVR12 rate (95% CI) |
|
| Age at baseline (years) 65="" years=""> ≥ 65 years |
95.2% (257/270) 100% (7/7) |
80.5% (210/261) 78.6% (11/14) |
14.7% (9.3%, 20.4%) 21.4% (-21.3%, 50.8%) |
| Sex Male Female |
93.5% (257/270) 98.1% (105/107) |
75.9% (132/174) 88.1% (89/101) |
17.7% (10.1%, 25.4%) 10.0% (3.2%, 18.1%) |
| Race White Black or African American Other |
95.2% (238/250) 100% (3/3) 95.8% (23/24) |
78.2% (187/239) 100% (1/1) 94.1% (32/34) |
17.0% (11.1%, 23.1%) 0.0% (-70.8%, 97.5%) 1.7% (-15.7%, 16.0%) |
SOF = sofosbuvir; RBV = ribavirin
Adapted from FDA Statistical Review
Table 9. Subgroup analysis of SVR12 for Trial 4*
| EPCLUSA + RBV 12 Weeks | ||
|---|---|---|
| SVR12 rate | 95% CI | |
| Age (years) 65="" years=""> ≥ 65 years |
95.9% (71/74) 84.6% (11/13) |
(88.6%, 99.2%) (54.6%, 98.1%) |
| Sex Men Women |
92.4% (61/66) 100% (21/21) |
(83.2%, 97.5%) (83.9%, 100%) |
| Race White Black or African American Other |
93.7% (74/79) 100% (5/5) 100% (3/3) |
(85.8%, 97.9%) 47.8%, 100%) (29.2%, 100%) |
*Because EPCLUSA with ribavirin for 12 weeks is the recommended dosage regimen, the results of the 12-and 24-week EPCLUSA treatment subgroups are not presented.
RBV = ribavirin
Adapted from FDA Statistical review
What are the possible side effects?
EPCLUSA may cause serious slowing of the heart rate in patients who have heart disease and are taking the medication, amiodarone.
The most common side effects of EPCLUSA when used alone are headache and tiredness.
The most common side effects of EPCLUSA when used with another approved HCV drug called ribavirin are tiredness, anemia, nausea, headache, difficulties sleeping and diarrhea.
What are the possible side effects (results of trials used to assess safety)?
Below is the summary of the most common adverse reactions (all grades with frequency of 5% or greater) from the three clinical trials (Trial 1, 2 and 3) in 1035 patients with HCV and either no cirrhosis or with compensated cirrhosis who received EPCLUSA for 12 weeks:
- Headache (21%)
- Fatigue (16%)
- Nausea (9%)
- Insomnia (5%)
FDA Clinical review
Below is the summary of the most common adverse reactions (all grades with frequency of 10% or greater) from the single clinical trial (Trial 4) in 87 patients with decompensated cirrhosis who received EPCLUSA with ribavirin for 12 weeks:
- Fatigue (32%),
- Anemia (26%),
- Nausea (15%),
- Headache (11%),
- Insomnia (11%), and
- Diarrhea (10%).
EPCLUSA Prescribing Information
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race, and age.
- Sex: The risk of side effects was similar in men and women.
- Race: The risk of side effects was similar in all races studied.
- Age: The risk of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the frequency of all adverse reactions for combined Trials 1, 2, and 3, by subgroup.
Table 10. Adverse Reactions in EPCLUSA Arm by Subgroup (Trials 1, 2 and 3)
| EPCLUSA N=1035 n(%) |
|
|---|---|
| Age (years) | |
| > | 465/912 (51%) |
| ≥65 | 55/123 (45%) |
| Sex | |
| Men | 302/630 (48%) |
| Women | 218/405 (54%) |
| Race | |
| White | 444/867 (51%) |
| Black or African American | 31/61 (51%) |
| Other* | 41/102 (40%) |
* Patients with 'Not reported' race are excluded.
Clinical trial data
The table below summarizes the frequency of all adverse reactions in EPCLUSA+ribavirin 12 week arm from Trial 4 by subgroup.
Table 11. Adverse Reactions in EPCLUSA+ribavirin 12 week Arm by Subgroup (Trial 4)
| EPCLUSA+RBV N=87 n(%) |
|
|---|---|
| Age (Years) | |
| > | 49/74 (66%) |
| ≥65 | 11/13 (85%) |
| Sex | |
| Men | 45/66 (68%) |
| Women | 15/21 (71%) |
| Race | |
| White | 55/79 (70%) |
| Black or African American | 2/5 (40%) |
| Other | 3/3 (100%) |
Clinical trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved EPCLUSA based on evidence from four clinical trials of 1825 patients with chronic hepatitis C virus infection. In these trials, some patients were previously treated for hepatitis C and some were never treated before. Some patients had cirrhosis and some did not. The trials were conducted in the United States, Puerto Rico, Canada, Europe, Australia, New Zealand and China.
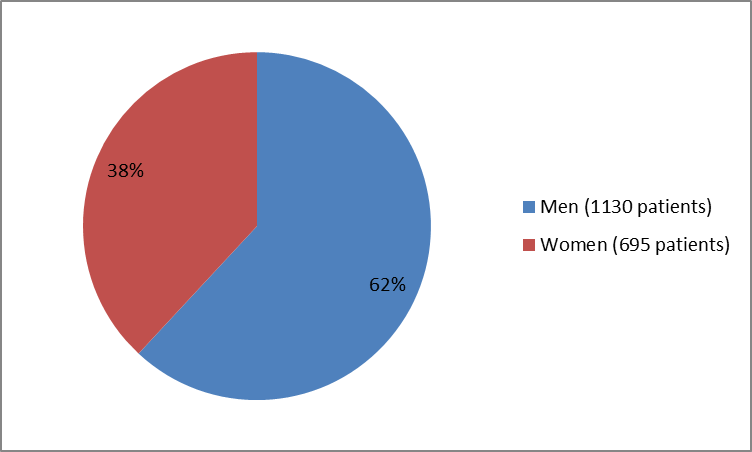
The figure below summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
Clinical trial data
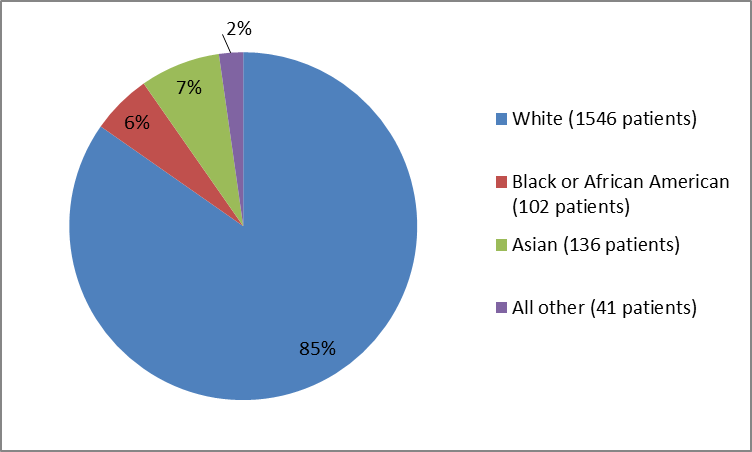
Figure 2 and Table 1 summarize the percentage of patients in the clinical trials.
Figure 2. Baseline Demographics by Race
Clinical trial data
Table 1. Demographics of Trials by Race
| Race | Number of Patients | Percentage |
| White | 1546 | 85% |
| Black or African American | 102 | 6% |
| Asian | 136 | 7% |
| American Indian or Alaska Native | 12 | 1% |
| Hawaiian or Pacific Islander | 6 | less than 1% |
| Other | 15 | 1% |
| Not reported | 8 | less than 1% |
Clinical trial data
Figure 3 summarizes the percentage of patients by age in the clinical trials.
Figure 3. Baseline Demographics by Age
Clinical trial data
Who participated in the trials?
The table below summarizes demographics of patients without cirrhosis or with compensated cirrhosis enrolled in the 3 clinical trials.
Table 12. Demographic Characteristics of Patients without Cirrhosis or with Compensated Cirrhosis (Trials 1, 2 and 3 -Safety Population)
| Characteristic | EPCLUSA 12 Week (N = 1035) |
Placebo 12 Week (N = 116) |
SOF+RBV 12 Week (N = 132) |
SOF+RBV 24 Week (N = 275) |
Total (N = 1558) |
|---|---|---|---|---|---|
| Age (years) | |||||
| Mean (SD) | 53 (11.0) | 53 (10.4) | 57 (9.3) | 50 (10.0) | 53 (10.7) |
| Min, Max | 18,82 | 25,74 | 23,76 | 19,74 | 18,82 |
| Age Group | |||||
| 65=""> | 912 (88.1%) | 104 (89.7%) | 110 (83.3%) | 261 (94.9%) | 1387 (89.0%) |
| ≥ 65 years | 123 (11.9%) | 12 (10.3%) | 22 (16.7%) | 14 (5.1%) | 171 (11.0%) |
| Sex n (%) | |||||
| Men | 630 (60.9%) | 68 (58.6%) | 72 (54.5%) | 174 (63.3%) | 944 (60.6%) |
| Women | 405 (39.1%) | 48 (41.4%) | 60 (45.5%) | 101 (36.7%) | 614 (39.4%) |
| Race, n (%) | |||||
| White | 867 (83.8%) | 90 (77.6%) | 111 (84.1%) | 239 (86.9%) | 1307 (83.9%) |
| Black or African American |
61 (5.9%) | 11 (9.5%) | 12 (9.1%) | 1 (0.4%) | 85 (5.5%) |
| Asian | 86 (8.3%) | 11 (9.5%) | 5 (3.8%) | 29 (10.5%) | 131 (8.4%) |
| American Indian/Alaska Native |
8 (0.8%) | 0 | 0 | 3 (1.1%) | 11 (0.7%) |
| Hawaiian or Pacific Islander | 1 (> | 1 (0.9%) | 1 (0.8%) | 2 (0.7%) | 5 (0.3%) |
| Other | 7 (0.7%) | 3 (2.6%) | 2 (1.5%) | 0 | 12 (0.8%) |
| Not reported | 5 (0.5%) | 0 | 1 (0.8%) | 1 (0.4%) | 7 (0.4%) |
| Ethnicity, n(%) | |||||
| Hispanic or Latino | 68 (6.6%) | 5 (4.3%) | 23 (17.4%) | 11 (4%) | 107 (6.9%) |
| Non Hispanic or Latino | 959 (92.7%) | 111 (95.7%) | 107 (81.1%) | 263 (95.6%) | 1440 (92.4%) |
| Not disclosed | 8 (0.8%) | 0 | 2(1.5%) | 1(0.4%) | 11(0.7%) |
| Region n(%) | |||||
| US | 428 (41.4%) | 45(38.8%) | 132 (100%) | 60 (21.8%) | 665(42.7%) |
| Non US | 607 (58.6%) | 71(61.2%) | 0 | 215 (78.2%) | 893 (57.3% |
SOF=sofosbuvir; RBV=ribavirin
Clinical trial data
The table below summarizes demographics of patients with decompensated cirrhosis enrolled in the single clinical trial.
Table 13. Demographic Characteristics of Patients with Decompensated Cirrhosis (Trial 4-Safety Population)
| Characteristics | EPCLUSA 1 2 Weeks* ( N = 90) |
EPCLUSA+RBV 1 2 Weeks ( N = 87) |
EPCLUSA 2 4 Weeks* ( N = 90) |
Total ( N = 267) |
||||
|---|---|---|---|---|---|---|---|---|
| Age (years) | ||||||||
| Mean (SD) | 58 (6.3) | 58 (6.9) | 58 (5.8) | 58 (6.3) | ||||
| Min, Max | 42,73 | 40,71 | 46,72 | 40,73 | ||||
| Age Group | ||||||||
| 65=""> | 81 (90.0%) | 74 (85.1%) | 79 (87.8%) | 234 (87.6%) | ||||
| ≥ 65 Years | 9 (10.0%) | 13 (14.9%) | 11 (12.2%) | 33 (12.4%) | ||||
| Sex, n (%) | ||||||||
| Men | 57 (63.3%) | 66 (75.9%) | 63 (70.0%) | 186 (69.7%) | ||||
| Women | 33 (36.7%) | 21 (24.1%) | 27 (30.0%) | 81 (30.3%) | ||||
| Race, n (%) | ||||||||
| White | 79 (87.8%) | 79 (90.8%) | 81 (90.0%) | 239 (89.5%) | ||||
| Black or African American | 6 (6.7%) | 5 (5.7%) | 6 (6.7%) | 17 (6.4%) | ||||
| Asian | 3 (3.3%) | 0 | 2 (2.2%) | 5 (1.9%) | ||||
| American Indian/ Alaska Native | 0 | 1 (1.1%) | 0 | 1 (0.4%) | ||||
| Hawaiian or Pacific Islander | 0 | 1 (1.1%) | 0 | 1 (0.4%) | ||||
| Other | 2 (2.2%) | 1 (1.1%) | 0 | 3 (1.1%) | ||||
| Not reported | 0 | 0 | 1 (1.1%) | 1 (0.4%) | ||||
| Ethnicity, n (%) | ||||||||
| Hispanic or Latino | 13 (14.4%) | 13 (14.9%) | 13 (14.4%) | 39 (14.6%) | ||||
| Not Hispanic or Latino | 77 (85.6%) | 74 (85.1%) | 77 (85.6%) | 228 (85.4%) | ||||
RBV=ribavirin
*Non-approved regimen
Clinical trial data
How were the trials designed?
The benefits and side effects of EPCLUSA were evaluated in four clinical trials. Each trial was designed differently.
In Trial 1, patients randomly received either EPCLUSA or placebo pill for 12 weeks. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed.
In Trial 2, patients randomly received either EPCLUSA for 12 weeks or the combination of two previously approved drugs called sofosbuvir and ribavirin for 12 weeks. In this trial, both patients and health care providers knew which treatment was given.
The Trial 3, patients randomly received EPCLUSA for 12 weeks or the combination of two previously approved drugs called sofosbuvir and ribavirin for 24 weeks. In this trial, both patients and health care providers knew which treatment was given.
In Trial 4, patients randomly received either EPCLUSA for 12 weeks, or EPCLUSA for 24 weeks, or EPCLUSA and a previously approved drug called ribavirin for 12 weeks. In this trial, both patients and health care providers knew which treatment was given. This was the only trial where patients also had to have advanced cirrhosis in order to participate.
All trials measured the blood level of hepatitis virus C before and after treatment.
How were the trials designed?
The efficacy and safety of EPCLUSA were established in 4 clinical trials with a total of 1825 adult patients who had chronic hepatitis C genotypes 1-6 infection. Across the trials, different populations were studied: treatment naïve, treatment experienced, with no cirrhosis or with cirrhosis (both compensated and decompensated).
Trial 1 was a randomized, multicenter, double blind, placebo controlled 12 week trial comparing EPCLUSA to placebo in patients with chronic HCV infection, and genotypes 1, 2, 4, 5, or 6.
Trial 2 was a randomized, multicenter, open-label 12 week trial comparing EPCLUSA to sofosbuvir and ribavirin combination treatment in patients with genotype 2 HCV infection.
Trial 3 was a randomized, multicenter, open-label trial comparing 12-week treatment with EPCLUSA to 24-week treatment with sofosbuvir and ribavirin combination treatment in patients with genotype 3 HCV infection.
Trial 4 was randomized, multicenter, open label, three arm trial comparing EPCLUSA for 12 and 24 weeks with EPCLUSA plus ribavirin for 12 weeks in patients with HCV genotypes 1- 6 infection and decompensated cirrhosis.
In all trials, the primary efficacy outcome was sustained virologic response (SVR12) defined as HCV RNA less than lower limit of quantification 12 weeks after the cessation of treatment.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.