Drug Trials Snapshots: DOJOLVI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the DOJOLVI Package Insert for complete information.
DOJOLVI (triheptanoin)
doh-johl-vee
Ultragenyx Pharmaceutical Inc.
Approval date: June 30, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
DOJOLVI is a drug that provides calories and fatty acids for pediatric and adult patients with long-chain fatty acid oxidation disorder.
Long-chain fatty acid oxidation disorders (LC-FAOD) are a group of rare, genetic, life threatening disorders caused by defects in the enzymes needed to produce the energy from fatty acids. Patients with LC-FAODs can suffer from muscle pain, fatigue, and heart failure because their bodies break down muscle as a source of energy as fat cannot be used as an energy source.
How is this drug used?
DOJOLVI is a liquid mixed with meals or snacks four or more times per day.
What are the benefits of this drug?
After four months of treatment, patients who received DOJOLVI showed similar heart function as patients who received trioctanoin (a different source of calories and fatty acids).
What are the benefits of this drug?
The efficacy of DOJOLVI as a source of calories and fatty acids was evaluated in Trial 3 comparing triheptanoin (7-carbon chain fatty acid) with trioctanoin (8-carbon chain fatty acid).
Baseline cardiovascular function in both groups was normal and within test/retest variability normally observed in repeated echocardiograms. After 4 months, patients in both groups had similar cardiac function as evaluated by resting echocardiogram and treadmill ergometry.
In addition, there were no differences observed between the triheptanoin and trioctanoin groups in how patients tolerated each fatty acid or in the evaluation of multiple blood markers of metabolism.
DOJOLVI Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The trial that looked at the benefit of DOJOLVI was too small to determine if there were any differences in sex, race and age subgroups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The trial that looked at the efficacy of DOJOLVI was too small to determine if there were any differences in sex, race and age subgroups.
What are the possible side effects?
The most common side effects are abdominal pain, diarrhea, vomiting and nausea.
What are the possible side effects?
The adverse reactions from the trials are summarized below.
The most common adverse reactions to DOJOLVI reported in the pooled safety population of Trial 1 and Trial 2 were gastrointestinal (GI)-related, and included abdominal pain (abdominal discomfort, abdominal pain, abdominal distension, abdominal pain upper GI pain) [60%], diarrhea [44%], vomiting [44%], and nausea [14%]
DOJOLVI Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar among males and females.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 18 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of the adverse reactions by sex and age subgroup for the safety population. Race was not analyzed because of predominance of White patients.
Table 1. Subgroup Analysis of Adverse Events (safety population)
| Adverse Events | Sex | Age | ||
|---|---|---|---|---|
| Male N=41 |
Female N=38 |
<18 years N=58 |
≥18 years N=21 |
|
| At least one TEAE, n(%) | 40 (98) | 38 (100) | 58 (100) | 20 (95) |
| Grade ≥4, n(%) | 4 (10) | 2 (5) | 6 (10) | 0 |
| SAE, n(%) | 32 (78) | 31 (82) | 51 (88) | 12 (57) |
| Gastrointestinal | 35 (85) | 33 (87) | 53 (91) | 20 (95) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the trials?
The FDA approved DOJOLVI based on evidence from three clinical trials (Trial 1/NCT018863, Trial 2/NCT022141 and Trial 3/NCT01379625). The trials enrolled pediatric and adult patients with LC-FAOD. Trials 1 and 2 were conducted at 11 sites in the United States and United Kingdom, and Trial 3 was conducted at two sites in the United States.
The population from combined Trials 1 and 2 -which provided data for side effects evaluation (safety population) - is presented below. Demographics of the population from Trial 3 - which provided data for benefit evaluation (efficacy population) -is presented in Table 3, under the MORE INFO section.
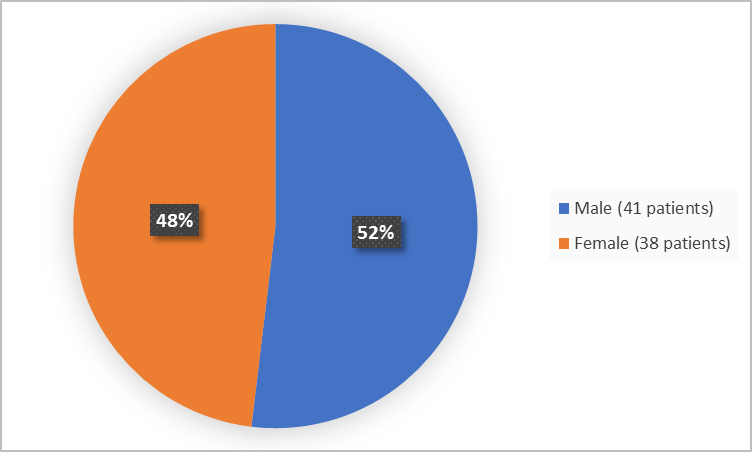
Figure 1 summarizes the percentage of patients by sex in the clinical trials used to evaluate the side effects.
Figure 1. Baseline Demographics by Sex (safety population)
FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trials used to evaluate the side effects.
Figure 2. Baseline Demographics by Race (safety population)
FDA Review
Figure 3 summarizes the percentage of patients by age in the clinical trials used to evaluate the side effects.
Figure 3. Baseline Demographics by Age (safety population)
FDA Review
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trials used to evaluate the side effects.
Figure 4. Baseline Demographics by Ethnicity (safety population)
FDA Review
Who participated in the trials?
The tables below summarize demographics of patients in safety and efficacy populations, respectively.
Table 2. Demographic Characteristics for Trials 1 and 2 (safety population)
| Demographic Characteristic | DOJOLVI N = 79 |
|---|---|
| Sex, n (%) | |
| Male | 41 (52) |
| Female | 38 (48) |
| Race, n (%) | |
| White | 69 (87) |
| Black or African American | 4 (5) |
| Asian | 3 (4) |
| Other | 3 (4) |
| Age (years) | |
| Mean (SD) | 13.4 (13.2) |
| Median | 9 |
| Range | (0.3-63) |
| Age Group, n (%) | |
| <6 years | 29 (37) |
| 6-17 years | 29 (37) |
| Patients) ≥ 18 years | 21 (26) |
| Ethnicity, n 9(%) | |
| Hispanic or Latino | 7 (9) |
| Not Hispanic or Latino | 71 (90) |
| Unknown | 1 (1) |
| Region | |
| United States | 72 (91) |
| United Kingdom | 7 (9) |
FDA Review
Table 3. Demographic Characteristics for Trial 3 (efficacy population)
| Demographic Characteristic | Total N = 32 |
|---|---|
| Sex, n (%) | |
| Male | 12 (38) |
| Female | 20 (62) |
| Race, not collected | |
| Age | |
| Mean (SD) | 24.75 (14.3) |
| Median | 23 |
| Range | 7, 64 |
| Age Group, n(%) | |
| <17 years | 13 (41) |
| ≥ 18 years | 29 (59) |
| Ethnicity, not collected | |
| Region | |
| United States | 32 (100) |
FDA Review
How were the trials designed?
Trials 1 and Trial 2 were used to evaluate the side effects of DOJOLVI. Both trials enrolled pediatric and adult patients diagnosed with LC-FAOD. In Trial 1, patients received DOJOLVI for 78 weeks. Trial 2 enrolled patients from other trials who were already treated with DOJOLVI (including those from Trial 1) as well as patients who were never treated with DOJOLVI before. Trial 2 is still ongoing and is planned to last up to 5 years.
The benefit of DOJOLVI was evaluated in Trial 3 which enrolled pediatric and adult patients with LC-FAOD. Half of the patients received DOJOLVI and half received trioctanoin for 4 months. Neither the patients nor the investigators knew which treatment was given until the end of the trial. The benefit of DOJOLVI in comparison to trioctanoin was assessed by measuring the changes in heart and muscle function.
How were the trials designed?
The FDA efficacy of DOJOLVI was established in a 4-month double-blind, randomized, controlled trial (Trial 3) comparing triheptanoin (7-carbon chain fatty acid) with trioctanoin (8-carbon chain fatty acid). The trial enrolled adult and pediatric patients with a confirmed diagnosis of LC-FAOD. After 4 months, patients in both groups were compared for both cardiac function and metabolic stability as assessed by clinical exam , cardiac imaging and laboratory testing of markers of blood metabolism and muscle breakdown.
Safety evaluation was based on data from two additional trials. Trial 1 was a phase 2, single arm, multicenter, prospective study to evaluate the impact of triheptanoin in patients 6 years of age and older who had been previously diagnosed with serious clinical manifestations of LC-FAOD despite current management. Patients were treated for up to 78 weeks. Trial 2 was an open-label, single arm, multicenter trial in patients who rolled over from previous commercial or investigator-sponsored trials using triheptanoin or in patients who were triheptanoin treatment-naïve. The patients were to be evaluated every 3 months for up to 5 years. The trial is currently ongoing.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION