Drug Trials Snapshots: CAPLYTA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the CAPLYTA Package Insert for complete information.
CAPLYTA (lumateperone)
Kah-PLY’-tah
Intra-Cellular Therapies, Inc.
Approval date: December 20, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
CAPLYTA is a drug used for the treatment of schizophrenia in adults.
Schizophrenia is a brain disorder with symptoms that include hearing voices, believing that other people are reading one’s mind or controlling their thoughts, and being suspicious or withdrawn.
How is this drug used?
CAPLYTA is a capsule that is taken once a day.
What are the benefits of this drug?
CAPLYTA improved symptoms of schizophrenia.
What are the benefits of this drug?
Table 1 summarizes the main results of Trials 1 and 2. Symptoms of schizophrenia were measured as the change in the Positive and Negative Syndrome Scale (PANSS) total score after 4 weeks. The table shows the results for all patients who had a baseline PANSS measurement, who had at least one measured PANSS Score after the baseline measurement, and who received at least four doses of the trial medication.
Table 1.Primary Efficacy Results for Change from Baseline in PANSS Total Score in Patients with Schizophrenia (Trials 1 and 2)
|
Trial |
Treatment Group |
N |
Primary Efficacy Endpoint: PANSS Total |
||
|---|---|---|---|---|---|
|
Mean Baseline Score (SD) |
LS Mean Change from Baseline (SE) |
Placebo-subtracted Difference (95% CI) |
|||
|
1 |
CAPLYTA * |
84 |
88.1 (11.0) |
-13.2 (1.7) |
-5.8 (-10.5, -1.1) a |
|
Placebo |
85 |
86.3 (13.1) |
-7.4 (1.7) |
-- |
|
|
2 |
CAPLYTA * |
150 |
90.0 (9.6) |
-14.5 (1.3) |
-4.2 (-7.8, -0.6) |
|
Placebo |
150 |
89.0 (10.3) |
-10.3 (1.3) |
-- |
|
|
The PANSS total score may range from 30 to 210; higher scores reflect greater symptom severity. SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval. aDifference (drug minus placebo) in LS mean change from baseline not adjusted for sample size increase after unblinded interim analysis. *Statistically significantly superior to placebo. |
|||||
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: CAPLYTA worked similarly in men and women.
- Race: CAPLYTA worked similarly in Black or African American and White patients.
- Age: CAPLYTA worked similarly in patients younger and older than 40 years of age. There were no patients older than 60 years; therefore, it is not known if there are any differences in how well CAPLYTA works in older patients.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Tables 2, 3, 4, and 5 summarize responses to CAPLYTA in the two trials by subgroup (sex and race). The subgroup analysis by race excluded races other than “Black or African American” and “White” because there were too few patients in the other racial categories to analyze.
Table 2. Subgroup Analysis of Primary Endpoint by Sex – Trial 1
|
Subgroup/ Treatment Group |
Efficacy Measure: Change from Baseline in PANSS Total Score to Day 28 |
||
|---|---|---|---|
|
Mean Baseline Score (SD) |
LS Mean Change from Baseline (SE) |
Placebo-subtracted Differencea (95% CI) |
|
|
Women
|
|
|
|
|
CAPLYTA (N=15) |
83.3 (9.9) |
-19.8 (3.8) |
-12.0 (-22.0, -2.0) |
|
Placebo (N=18) |
89.7 (10.5) |
-7.8 (3.4) |
-- |
|
Men
|
|
|
|
|
CAPLYTA (N=61) |
89.3 (11.0) |
-11.4 (1.8) |
-4.0 (-9.3, 1.2) |
|
Placebo (N=62) |
85.3 (13.7) |
-7.4 (1.9) |
-- |
|
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval. a Difference (drug minus placebo) in least-squares mean change from baseline. |
|||
Table 3. Subgroup Analysis of Primary Endpoint by Race – Trial 1
|
Subgroup/ Treatment Group |
Efficacy Measure: Change from Baseline in PANSS Total Score to Day 28 |
||
|---|---|---|---|
|
Mean Baseline Score (SD) |
LS Mean Change from Baseline (SE) |
Placebo-subtracted Differencea (95% CI) |
|
|
Black or African American
|
|
|
|
|
CAPLYTA (N=63) |
88.2 (11.2) |
-13.6 (1.8) |
-3.6 (-8.8, 1.7) |
|
Placebo (N=61) |
85.5 (12.1) |
-10.0 (1.9) |
-- |
|
White |
|
|
|
|
CAPLYTA (N=12) |
88.5 (10.2) |
-9.9 (4.6) |
-10.6 (-22.4, 1.2) |
|
Placebo (N=16) |
89.4 (17.1) |
0.7 (3.9) |
-- |
|
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval. a Difference (drug minus placebo) in least-squares mean change from baseline.
|
|||
Table 4. Subgroup Analysis of Primary Endpoint by Sex – Trial 2
|
Subgroup/ Treatment Group |
Efficacy Measure: Change from Baseline in PANSS Total Score to Day 28 |
||
|---|---|---|---|
|
Mean Baseline Score (SD) |
LS Mean Change from Baseline (SE) |
Placebo-subtracted Differencea (95% CI) |
|
|
Women
|
|
|
|
|
CAPLYTA (N=35) |
90.6 (9.6) |
-15.2 (2.4) |
-5.0 (-12.6, 2.6) |
|
Placebo (N=21) |
88.8 (8.9) |
-10.2 (3.0) |
-- |
|
Men
|
|
|
|
|
CAPLYTA (N=108) |
90.0 (9.6) |
-14.2 (1.5) |
-3.9 (-8.0, 0.2) |
|
Placebo (N=115) |
90.1 (11.4) |
-10.3 (1.5) |
-- |
|
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval. a Difference (drug minus placebo) in least-squares mean change from baseline. |
|||
Table 5. Subgroup Analysis of Primary Endpoint by Race – Trial 2
|
Subgroup/ Treatment Group |
Efficacy Measure: Change from Baseline in PANSS Total Score to Day 28 |
||
|---|---|---|---|
|
Mean Baseline Score (SD) |
LS Mean Change from Baseline (SE) |
Placebo- subtracted Differencea (95% CI) |
|
|
Black or African American |
|
|
|
|
CAPLYTA (N=107) |
90.3 (9.5) |
-15.9 (1.5) |
-4.6 (-8.9, -0.3) |
|
Placebo (N=92) |
89.7 (11.6) |
-11.3 (1.6) |
-- |
|
White
|
|
|
|
|
CAPLYTA (N=32) |
89.4 (9.3) |
-12.0 (2.6) |
-3.1 (-10.3, 4.1) |
|
Placebo (N=38) |
91.3 (10.1) |
-8.9 (2.5) |
-- |
|
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval. a Difference (drug minus placebo) in least-squares mean change from baseline.
|
|||
What are the possible side effects?
CAPLYTA may cause serious side effects including:
- Increased risk of death in the elderly with dementia-related psychosis (elderly who have lost touch with reality [psychosis] due to confusion and memory loss [dementia]).
- Increased risk of stroke in elderly patients with dementia-related psychosis
- Neuroleptic syndrome (life threatening condition that includes fever, stiff muscles and altered mental status)
- Tardive dyskinesia (uncontrolled involuntary movements of the face, tongue, arms, legs, and other body parts)
- Weight gain and high blood glucose and lipids
- Low white blood cell counts
- Decrease in blood pressure upon standing and loss of consciousness
- Falls
- Seizures
- Impaired ability to operate machinery
The most common side effects of CAPLYTA are sleepiness and dry mouth.
What are the possible side effects?
Table 6 summarizes adverse reactions for the pool of three placebo-controlled trials (4- to 6-week dosing).
Table 6. Adverse Reactions Reported in >2% of Patients Treated with CAPLYTA and at a Greater Frequency than Placebo-treated Patients in 4-to 6-week Schizophrenia Trials
|
Adverse Reactions |
CAPLYTA (N=406) |
Placebo (N=412) |
|
|---|---|---|---|
|
Somnolence/ Sedation |
24% |
10% |
|
|
Nausea |
9% |
5% |
|
|
Dry Mouth |
6% |
2% |
|
|
Dizziness1 |
5% |
3% |
|
|
Creatine Phosphokinase Increased |
4% |
1% |
|
|
Liver Enzymes Increased2 |
2% |
1% |
|
|
Fatigue |
3% |
1% |
|
|
Vomiting |
3% |
2% |
|
|
Decreased Appetite |
2% |
1% |
|
|
1Dizziness, dizziness postural 2ALT, AST, “hepatic enzymes” increased, or liver function test abnormal |
|||
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The risk of overall side effects was similar in Black or African American and White patients.
- Age: The occurrence of side effects was similar in patients younger and older than 40 years of age. There were no patients older than 60 years; therefore, it is not known if the side effects in older patients are similar to those in younger patients.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below shows the number and percentage of patients with at least one treatment-emergent adverse event by subgroup in the pooled trials.
Table 7. Subgroup Analysis of Adverse Events in the Pooled Trials
|
|
CAPLYTA N=406
|
Placebo N=412
|
|---|---|---|
|
Sex, n (%) |
|
|
|
Men |
193/299 (65) |
174/320 (54) |
|
Women |
74/107 (69) |
55/92 (60) |
|
Race, n (%) |
|
|
|
Black or African American African Descent |
207/318 (65) |
156/294 (53) |
|
White |
51/76 (67) |
57/94 (61) |
|
All Other |
9/12 (75) |
16/24 (67) |
|
Age Group, n (%) |
|
|
|
≤ 40 years |
114/185 (62) |
80/176 (45) |
|
> 40 years |
153/221 (69) |
149/236 (63) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the trials?
The FDA approved CAPLYTA based on evidence from three clinical trials (Trial 1/NCT01499563, Trial 2/NCT02282761 and Trial 3/NCT02469155) that enrolled 818 adult patients with schizophrenia. The trials were conducted at 33 sites in the United States.
Trials 1 and 2 provided data on the benefits and side effects of CAPLYTA, and Trial 3 provided data on side effects only.
The figure below summarizes the numbers of men and women who participated in the three clinical trials and received at least one dose of trial drug (safety population).
Figure 1. Demographics by Sex (safety population)
Clinical Trial Data
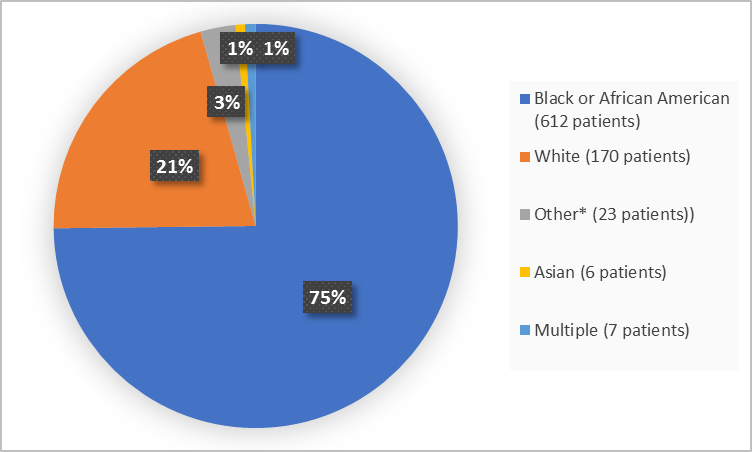
The figure below summarizes how many patients participated in the clinical trials by race.
Figure 2. Demographics by Race (safety population)
*Includes Other, American Indian or Alaska Native and Native Hawaiian or Other Pacific Islander
Clinical Trial Data
The figure below summarizes how many patients participated in the clinical trials by age.
Figure 3. Demographics by Age (safety population)
Clinical Trial Data
Who participated in the trials?
The table below summarizes demographics for the patients who participated in the three trials and were treated with either CAPLYTA 42 mg or placebo.
Table 8. Demographic Characteristics (safety population)
|
|
CAPLYTA N=406
|
Placebo N=412
|
TOTAL N=818 |
|---|---|---|---|
|
Sex, n (%) |
|
|
|
|
Men |
299 (73.6) |
320 (77.7) |
619 (75.7) |
|
Women |
107 (26.4) |
92 (22.3) |
199 (24.3) |
|
Race, n (%) |
|
|
|
|
Black or African American African Descent |
318 (78.3) |
294 (71.4) |
612 (74.8) |
|
White |
76 (18.7) |
94 (22.8) |
170 (20.8) |
|
Other |
6 (1.5) |
14 (3.4) |
20 (2.4) |
|
Multiple |
3 (0.7) |
4 (1.0) |
7 (0.9) |
|
Asian |
1 (0.2) |
5 (1.2) |
6 (0.7) |
|
American Indian or Alaska Native |
1 (0.2) |
1 (0.2) |
2 (0.2) |
|
Native Hawaiian or Other Pacific Islander |
1 (0.2) |
0 |
1 (0.1) |
|
Age (years) |
|
|
|
|
Median |
43 |
44 |
43 |
|
Min, Max |
20, 60 |
18, 60 |
18, 60 |
|
Age Group, n (%) |
|
|
|
|
≤ 40 years |
185 (45.6) |
176 (42.7) |
361 (44.1) |
|
> 40 years |
221 (56.4) |
236 (57.3) |
457 (55.9) |
|
<65 years |
406 (100) |
412 (100) |
818 (100) |
|
Ethnicity |
|
|
|
|
Hispanic or Latino |
30 (7.4) |
45 (10.9) |
75 (9.2) |
|
Not Hispanic or Latino |
376 (92.6) |
367 (89.1) |
743 (90.8) |
|
Region |
|
|
|
|
USA |
406 (100) |
412 (100) |
818 (100) |
Clinical Trial Data
How were the trials designed?
Three trials provided data for CAPLYTA’s approval. In each trial, hospitalized patients with schizophrenia were randomly assigned to receive either CAPLYTA or a comparison treatment (placebo or active comparator) once daily for 4 weeks (Trials 1 and 2) or 6 weeks (Trial 3). Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
Trials 1 and 2 provided data for the assessment of benefits and side effects through 4 weeks of therapy. Benefit was assessed by measuring the overall improvement in the symptoms of schizophrenia.
Trial 3 provided data for the assessment of side effects only during 6 weeks of therapy.
How were the trials designed?
Three randomized, double-blind, placebo-controlled, multi-center trials were conducted in adult patients who met Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for schizophrenia. All trials were conducted in hospital units in the United States and enrolled patients experiencing an acute exacerbation of schizophrenia. For all trials, neither the patients nor the health care providers knew which treatment was given until after the trial was completed.
In Trial 1, patients were randomized to receive CAPLYTA 42 mg, CAPLYTA 84 mg, an active comparator, or placebo for a four-week treatment period.
In Trial 2, patients were randomized to receive CAPLYTA 28 mg, CAPLYTA 42 mg, or placebo for a four-week treatment period.
In Trial 3, patients were randomized to receive CAPLYTA 14 mg, CAPLYTA 42 mg, an active comparator, or placebo for a six-week treatment period.
In all three trials, the primary endpoint was the change from baseline to the end of the treatment period in the Positive and Negative Syndrome Scale (PANSS) total score. The PANSS is a 30-item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); the total PANSS scores range from 30 to 210 with higher scores reflecting greater overall symptom severity.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION