Drug Trials Snapshots: BRIUMVI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the BRIUMVI Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
BRIUMVI (ublituximab-xiiy)
(bree-UM-vee)
TG Therapeutics, Inc.
Approval date: December 28, 2022
DRUG TRIALS SNAPSHOT SUMMARY
What is the drug for?
BRIUMVI is a monoclonal antibody that is used to treat relapsing forms of multiple sclerosis (MS) including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
In relapsing forms of MS (RMS), patients have episodes of worsening function (relapses) followed by recovery periods. Patients can also experience an increase in the underlying disability, particularly as the disease progresses.
How is this drug used?
BRIUMVI is an intravenous (IV) infusion that is taken every six months following an initial two doses taken two weeks apart. The first infusion is 150 mg, followed by a second infusion of 450 mg two weeks later, then subsequent infusions of 450 mg every 24 weeks.
Who participated in the clinical trials?
The FDA approved BRIUMVI based on evidence from two clinical trials (Studies 1 and 2) of 1,093 patients with RMS. The trials were conducted at 110 of sites in 10 countries in North America and Europe. The trials were used to assess both efficacy and safety of BRIUMVI.
How were the trials designed?
The benefits and side effects of BRIUMVI were evaluated in two clinical trials of patients with RMS. Patients received BRIUMVI or teriflunomide for up to 96 weeks. Neither the patients nor the health care providers knew which treatment was being given until the trials were completed.
The benefit of BRIUMVI was evaluated based on the annualized relapse rate (ARR), or the number of relapses per year, over the treatment period.
How were the trials designed?
BRIUMVI was evaluated in two clinical trials of 1,093 patients with RMS. These two clinical trials were 96-week double-blind, double-dummy, parallel group, active comparator-controlled clinical trials of identical design. The active comparator for these trials was teriflunomide, another product approved for the treatment of RMS. Patients were randomized to receive either BRIUMVI, given as an IV infusion of 150 mg for the first dose, 450 mg two weeks after the first infusion for the second dose, and 450 mg every 24 weeks after the first infusion for subsequent doses (third dose and beyond) with oral placebo administered daily; or teriflunomide, the active comparator, given orally as a 14 mg daily dose with IV placebo administered on the same schedule as BRIUMVI.
Both studies enrolled patients who had experienced at least one relapse in the previous year, two relapses in the previous two years, or had the presence of a T1 gadolinium (Gd)-enhancing lesion in the previous year. Patients were also required to have an Expanded Disability Status Scale (EDSS) score from 0 to 5.5 at baseline. Neurological evaluations were performed at baseline, every 12 weeks, and at the time of a suspected relapse. Brain magnetic resonance imaging (MRI) scans were performed at baseline and at Weeks 12, 24, 48, and 96.
The primary outcome of these studies was the ARR, or the number of relapses per year, over the treatment period.
DEMOGRAPHICS SNAPSHOT
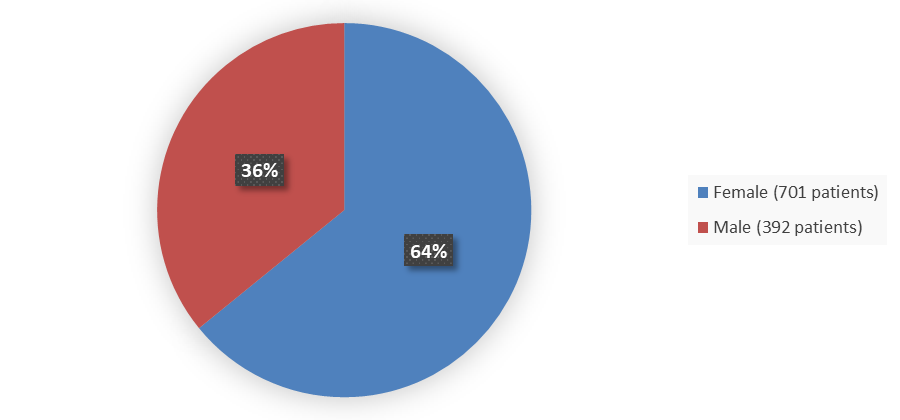
Figure 1. Baseline Demographics by Sex in the Combined BRIUMVI Phase 3 Clinical Trials
Source: Adapted from FDA Review
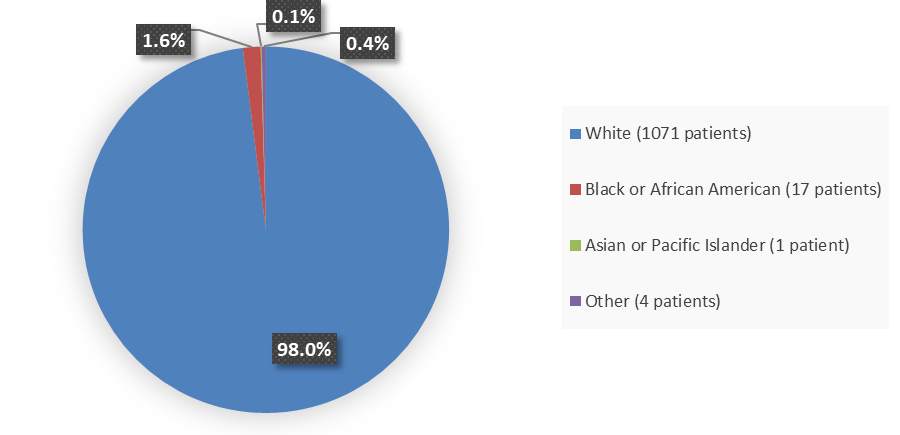
Figure 2. Baseline Demographics by Race in the Combined BRIUMVI Phase 3 Clinical Trials
Source: Adapted from FDA Review
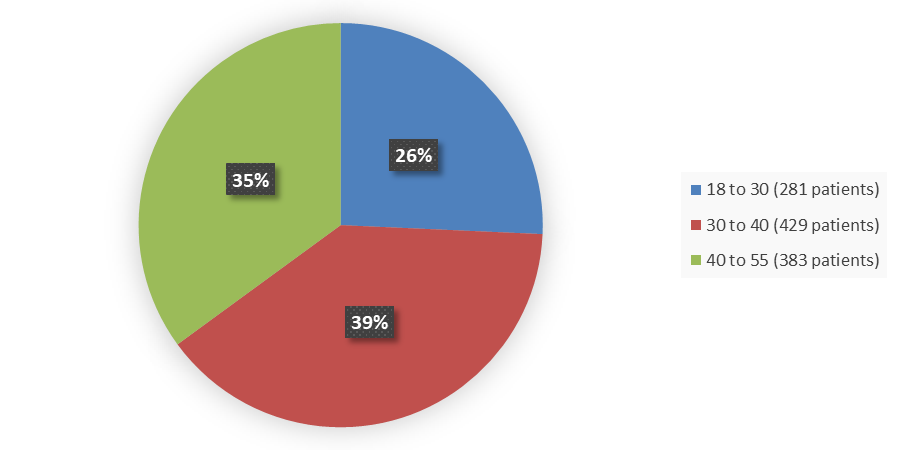
Figure 3. Baseline Demographics by Age in the Combined BRIUMVI Phase 3 Clinical Trials
Source: Adapted from FDA Review
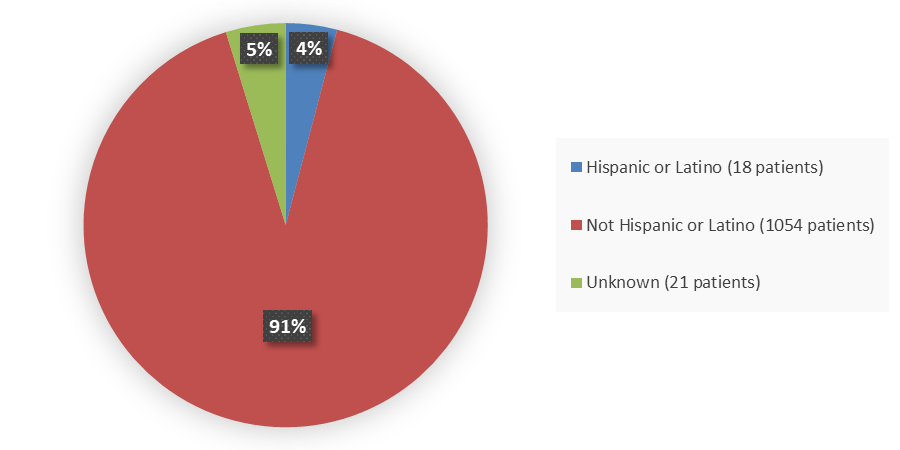
Figure 4. Baseline Demographics by Ethnicity in the Combined BRIUMVI Phase 3 Clinical Trials
Source: Adapted from FDA Review
Who participated in the trials?
Table 1 summarizes the demographics of patients in the combined BRIUMVI clinical trials in patients with relapsing forms of multiple sclerosis.
Table 1. Baseline Demographics in the Combined BRIUMVI Phase 3 Clinical Trials Safety Population
| Demographic | BRIUMVI N=545 n (%) |
Teriflunomide N=548 n (%) |
Total N=1093 n (%) |
|---|---|---|---|
| Sex | |||
| Female | 345 (63.3) | 356 (65.0) | 701 (64.1) |
| Male | 200 (36.7) | 192 (35.0) | 392 (35.9) |
| Age (years) | |||
| Mean (SD) | 35.4 (8.6) | 36.6 (9.3) | 36.0 (9.0) |
| Median (IQR) | 35.0 (14.0) | 36.0 (14.0) | 36.0 (14.0) |
| Min, max | 18, 55 | 18, 55 | 18, 55 |
| Race | |||
| White | 535 (98.2) | 536 (97.8) | 1071 (98.0) |
| Black or African American | 8 (1.5) | 9 (1.6) | 17 (1.6) |
| Other | 1 (0.2) | 3 (0.6) | 4 (0.4) |
| Native Hawaiian or other Pacific Islander | 1 (0.2) | 0 (0) | 1 (0.1) |
| Ethnicity | |||
| Not Hispanic or Latino | 524 (96.2) | 530 (96.7) | 1054 (96.4) |
| Hispanic or Latino | 13 (2.4) | 5 (0.9) | 18 (1.7) |
| Not reported or unknown | 8 (1.5) | 13 (2.4) | 21 (1.9) |
| Region | |||
| United States | 40 (7.3) | 44 (8.0) | 84 (7.7) |
| Rest of world | 505 (92.7) | 504 (92.0) | 1009 (92.3) |
| Eastern Europe | 492 (90.3) | 495 (90.3) | 987 (90.3) |
| Western Europe | 13 (2.4) | 9 (1.6) | 22 (2.0) |
Source: Adapted from FDA Review
Abbreviations: IQR, interquartile ratio; SD, standard deviation
What are the benefits of this drug?
BRIUMVI was better in reducing the risk of relapse in comparison to teriflunomide, a product approved for the treatment of relapsing forms of MS.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy of BRIUMVI was demonstrated in two randomized, double-blind, double-dummy, parallel group, active comparator-controlled clinical trials of identical design, in patients with RMS treated for 96 weeks. The primary outcome of these studies was the ARR over the treatment period. BRIUMVI significantly lowered the ARR compared to teriflunomide, another therapy approved for the treatment of RMS (Table 2). Additionally, BRIUMVI significantly reduced the number of new MRI lesions (both T1 Gd enhancing lesions and T2 hyperintense lesions) compared to teriflunomide.
However, BRIUMVI did not reduce the proportion of patients with 12-week confirmed disability progression compared to teriflunomide.
Table 2. Key Clinical and MRI Endpoints in RMS Patients From BRIUMVI Clinical Trials
| Endpoint | Study 1 | Study 2 | |||||
|---|---|---|---|---|---|---|---|
| BRIUMVI 450 mg7 |
Teriflunomide 14 mg7 |
BRIUMVI 450 mg7 |
Teriflunomide 14 mg7 |
||||
| Clinical endpoints1 | |||||||
| Annualized relapse rate (primary endpoint) | 0.076 | 0.188 | 0.091 | 0.178 | |||
| Relative reduction | 59% (p<0.001) | 49% (p=0.002) | |||||
| Proportion of patients with 12-week confirmed disability progression2,3 | 5.2% BRIUMVI vs. 5.9% teriflunomide | ||||||
| Risk reduction (pooled analysis)4 | 16% (p=0.510) | ||||||
| MRI endpoints5 | |||||||
| Mean number of T1 Gd-enhancing lesions per MRI6 | 0.016 | 0.491 | 0.009 | 0.250 | |||
| Relative reduction | 97% (p<0.001) | 97% (p<0.001) | |||||
| Mean number of new or enlarging T2 hyperintense lesions per MRI6 | 0.213 | 2.789 | 0.282 | 2.831 | |||
| Relative reduction | 92% (p<0.001) | 90% (p<0.001) | |||||
Source: BRIUMVI Prescribing Information
1 Based on modified intent-to-treat (mITT) population, defined as all randomized patients who received at least one infusion of study medication and had one baseline and postbaseline efficacy assessment. Study 1: BRIUMVI (N=271), teriflunomide (N=274). Study 2: BRIUMVI (N=272), teriflunomide (N=272).
2 Data prospectively pooled from Study 1 and Study 2: BRIUMVI (N=543), teriflunomide (N=546).
3 Defined as an increase of 1.0 point or more from the baseline EDSS score for patients with baseline score of 5.5. or less, or 0.5 point or more when the baseline score is greater than 5.5, Kaplan-Meier estimates at Week 96.
4 Based on hazard ratio.
5 Based on MRI-mITT population (mITT patients who have baseline and postbaseline MRI). Study 1: BRIUMVI (N=265), teriflunomide (N=270). Study 2: BRIUMVI (N=272), teriflunomide (N=267).
6 At Week 96.
7 BRIUMVI dosing by intravenous infusion: first dose of 150 mg, second dose 450 mg two weeks after the first; subsequent doses 450 mg every 24 weeks; teriflunomide dosing: 14 mg by mouth once daily.
Abbreviations: EDSS, Expanded Disability Status Scale; Gd, gadolinium; MRI, magnetic resonance imaging
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: BRIUMVI worked similarly in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in how BRIUMVI worked among races could not be determined.
- Age: No patients above 65 years of age were enrolled in the clinical trials of BRIUMVI. BRIUMVI worked similarly in patients below and above 38 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The reduction in relapses associated with BRIUMVI appeared similar among sex and age groups. The rate ratio for ARR was generally similar across subgroups based on sex and age category (Table 3). Meaningful subpopulation analysis by race was precluded by the small number of participants other than White enrolled in the clinical studies.
Table 3. Efficacy of BRIUMVI Across Subgroups of Sex and Age
| Subgroup | Study 1 | Study 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BRIUMVI | Teriflunomide | Rate Ratio | BRIUMVI | Teriflunomide | Rate Ratio | |||||
| N | ARR | N | ARR | N | ARR | N | ARR | |||
| Sex | ||||||||||
| Female | 166 | 0.104 | 179 | 0.175 | 0.594 | 178 | 0.093 | 176 | 0.179 | 0.523 |
| Male | 105 | 0.038 | 95 | 0.188 | 0.201 | 94 | 0.085 | 96 | 0.172 | 0.493 |
| Age category, years | ||||||||||
| <38 | 150 | 0.079 | 146 | 0.336 | 0.235 | 176 | 0.083 | 156 | 0.206 | 0.401 |
| ≥38 | 121 | 0.075 | 128 | 0.082 | 0.907 | 96 | 0.101 | 116 | 0.135 | 0.750 |
Source: Adapted from FDA Review
Abbreviations: ARR, annualized relapse rate
What are the possible side effects?
BRIUMVI can cause serious side effects, including:
- Infusion reactions, which are one of the most common side effects. Infusion reactions can be serious and may require you to be hospitalized. Infusion reaction symptoms can include fever, chills, headache, flu-like symptoms, fast heartbeat, hives, itchy skin, feeling faint, trouble breathing, and swelling of the tongue or throat.
- Infections are another common side effect of BRIUMVI. BRIUVMI increases the risk of getting infections caused by bacteria or viruses that may be life-threatening or cause death. Upper and lower respiratory tract infections are one of the most common side effects of BRIUMVI. Serious infections that may happen with BRIUMVI include hepatitis B virus reactivation and progressive multifocal leukoencephalopathy, a rare, serious brain infection that may cause death or severe disability.
- Low immunoglobulins, or a decrease in some types of antibodies, can result from treatment with BRIUMVI.
What are the possible side effects (results of trials used to assess safety)?
In active-controlled clinical trials, 545 patients with RMS received BRIUMVI. The most common adverse reactions in RMS trials (incidence of at least 10%) were infusion reactions and upper respiratory tract infections. Table 4 summarizes the adverse reactions that occurred in RMS trials (Study 1 and Study 2). The most common cause of discontinuation in patients treated with BRIUMVI was infection (1.3%).
Table 4. Adverse Reactions With an Incidence of at Least 5% for BRIUMVI and Higher Than Teriflunomide From Combined Phase 3 Studies
| Adverse Reaction | BRIUMVI 450 mg IV1 N=545 % |
Teriflunomide 14 mg PO N=548 % |
|---|---|---|
| Infusion reactions | 48 | 12 |
| Upper respiratory tract infections2 | 45 | 41 |
| Lower respiratory tract infections3 | 9 | 7 |
| Herpes virus-associated infections4 | 6 | 5 |
| Pain in extremity | 6 | 4 |
| Insomnia | 6 | 3 |
| Fatigue | 5 | 4 |
Source: BRIUMVI Prescribing Information
1 The first dose of BRIUMVI was given as an intravenous (IV) infusion of 150 mg. The second dose was given as an IV infusion of 450 mg two weeks after the first infusion.
2 Includes the following: nasopharyngitis, upper respiratory tract infections, respiratory tract infection, respiratory tract infection viral, pharyngitis, rhinitis, sinusitis, acute sinusitis, laryngitis, chronic sinusitis, viral pharyngitis, viral rhinitis, viral upper respiratory tract infection, chronic tonsillitis, pharyngitis streptococcal, sinusitis bacterial, and tonsillitis bacterial.
3 Includes the following: bronchitis, pneumonia, tracheitis, tracheobronchitis, COVID-19 pneumonia, bronchitis bacteria, and pneumonia viral.
4 Includes several related terms.
Abbreviations: IV, intravenous; PO, by mouth
The key safety issues are discussed in further detail below:
A major risk observed with BRIUMVI is that of infection. Serious and fatal infections occurred in BRIUMVI-treated patients during the controlled trials. Additionally, the COVID-19 pandemic that occurred during the Phase 3 trials for BRIUMVI led to observation of several serious and fatal cases of COVID-19 associated with BRIUMVI treatment. However, serious infections also have been reported with other anti-CD20 therapies approved for RMS.
Infusion-related reactions, including some serious reactions, were observed during BRIUMVI infusion. Patients enrolled in the clinical trials received premedication for infusion-related reaction prevention.
Decreased immunoglobulins are a known risk associated with anti-CD20 therapies, particularly with long-term use. A decrease in immunoglobulin M was observed in BRIUMVI-treated subjects during the controlled clinical trials, but in other anti-CD20 therapy development programs, reduction in immunoglobulin G did not occur until after years of treatment. Therefore, we did not expect to observe decreased immunoglobulin G in the clinical trial setting. A postmarketing requirement was issued to better characterize this risk with BRIUMVI and how it translates to risk of infection over time. Labeling instructs prescribers to monitor immunoglobulins during treatment with BRIUMVI.
BRIUMVI also may cause fetal harm. The nonclinical embryofetal safety results are concerning for a risk of embryofetal toxicity, in addition to that associated with B-cell depletion. The clinical data obtained during the RMS development program were insufficient to support conclusions about effects of BRIUMVI on human reproduction and pregnancy.
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: No patients above 65 years of age were enrolled in the clinical trials of BRIUMVI. The occurrence of side effects was similar overall in patients below and above 38 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Safety analyses by sex and age group were conducted in the combined Phase 3 studies. Meaningful subpopulation analysis by race was precluded by the small number of participants other than White enrolled in the clinical studies.
Review of treatment emergent adverse events by sex demonstrated that females on BRIUMVI were somewhat more likely to experience upper respiratory tract infections, urinary tract infections/cystitis, and symptoms typically associated with infusion-related reactions (e.g., headache, pyrexia, nausea) compared to males (Table 5). Males receiving BRIUMVI were more likely to experience chills. Otherwise, there did not appear to be any clear or clinically meaningful differences between males and females among subjects receiving BRIUMVI.
Table 5. Treatment-Emergent Adverse Events With an Incidence of at Least 5% for BRIUMVI From Combined Phase 3 Studies, by Sex
| Preferred Term | BRIUMVI | Teriflunomide | ||
|---|---|---|---|---|
| Female N=345 n (%) |
Male N=200 n (%) |
Female N=356 n (%) |
Male N=192 n (%) |
|
| Headache | 138 (40.0) | 49 (24.5) | 109 (30.6) | 37 (19.3) |
| Nasopharyngitis | 74 (21.4) | 26 (13.0) | 65 (18.3) | 33 (17.2) |
| Pyrexia | 57 (16.5) | 19 (9.5) | 20 (5.6) | 7 (3.6) |
| Nausea | 49 (14.2) | 9 (4.5) | 34 (9.6) | 9 (4.7) |
| Back pain | 40 (11.6) | 11 (5.5) | 35 (9.8) | 18 (9.4) |
| Diarrhea | 32 (9.3) | 12 (6.0) | 40 (11.2) | 18 (9.4) |
| Abdominal pain | 32 (9.3) | 11 (5.5) | 16 (4.5) | 5 (2.6) |
| Lymphopenia | 32 (9.3) | 21 (10.5) | 4 (1.1) | 2 (1.0) |
| Respiratory tract infection | 31 (9.0) | 11 (5.5) | 25 (7.0) | 13 (6.8) |
| Lymphocyte count decreased | 31 (9.0) | 18 (9.0) | 5 (1.4) | 5 (2.6) |
| Upper respiratory tract infection | 29 (8.4) | 12 (6.0) | 25 (7.0) | 13 (6.8) |
| Influenza like illness | 27 (7.8) | 12 (6.0) | 5 (3.1) | 0 (0) |
| Respiratory tract infection viral | 26 (7.5) | 16 (8.0) | 21 (5.9) | 10 (5.2) |
| Pharyngitis | 26 (7.5) | 6 (3.0) | 5 (3.1) | 1 (0.5) |
| Oropharyngeal pain | 24 (7.0) | 7 (3.5) | 17 (4.8) | 2 (1.0) |
| Cystitis | 23 (6.7) | 2 (1.0) | 20 (5.6) | 0 (0) |
| Bronchitis | 22 (6.4) | 2 (1.0) | 13 (3.7) | 5 (2.6) |
| Hyperthermia | 22 (6.4) | 9 (4.5) | 5 (1.4) | 1 (0.5) |
| Pain in extremity | 22 (6.4) | 9 (4.5) | 20 (5.6) | 4 (2.1) |
| Insomnia | 22 (6.4) | 11 (5.5) | 7 (2.0) | 9 (4.7) |
| Urinary tract infection | 20 (5.8) | 2 (1.0) | 24 (6.7) | 5 (2.6) |
| Cough | 20 (5.8) | 4 (2.0) | 8 (2.2) | 2 (1.0) |
| Infusion related reaction | 19 (5.5) | 8 (4.0) | 2 (0.6) | 1 (0.5) |
| Abdominal pain upper | 18 (5.2) | 2 (1.0) | 20 (5.6) | 3 (1.6) |
| Dizziness | 18 (5.2) | 6 (3.0) | 10 (2.8) | 7 (3.6) |
| Chills | 18 (5.2) | 26 (13.0) | 3 (0.8) | 1 (0.5) |
| Fatigue | 18 (5.2) | 10 (5.0) | 12 (3.4) | 8 (4.2) |
| Anxiety | 18 (5.2) | 6 (3.0) | 20 (5.6) | 3 (1.6) |
| Sinus tachycardia | 18 (5.2) | 7 (3.5) | 4 (1.1) | 6 (3.1) |
| Toothache | 12 (3.5) | 11 (5.5) | 17 (4.8) | 4 (2.1) |
| Rhinitis | 10 (2.9) | 14 (7.0) | 16 (4.5) | 5 (2.6) |
| Dyspepsia | 9 (2.6) | 14 (7.0) | 12 (3.4) | 8 (4.2) |
| Tachycardia | 9 (2.6) | 11 (5.5) | 12 (3.4) | 4 (2.1) |
Source: Adapted from FDA Review
Review of treatment-emergent adverse events by age (<38 years versus ≥38 years) demonstrated that certain preferred terms associated with respiratory tract infections (e.g., nasopharyngitis, rhinitis, bronchitis, and respiratory tract infection) were somewhat more common in the younger subpopulation receiving BRIUMVI (Table 6).
Otherwise, there did not appear to be any clear or clinically meaningful differences between the age subgroups among subjects receiving BRIUMVI.
Table 6. Treatment-Emergent Adverse Events With an Incidence of at Least 5% for BRIUMVI From Combined Phase 3 Studies, by Age
| Preferred Term | BRIUMVI | Teriflunomide | ||
|---|---|---|---|---|
| <38 Years N=326 n (%) |
≥38 Years N=219 n (%) |
<38 Years N=302 n (%) |
≥38 Years N=246 n (%) |
|
| Headache | 129 (39.6) | 58 (26.5) | 90 (29.8) | 56 (22.8) |
| Nasopharyngitis | 71 (21.8) | 29 (13.2) | 62 (20.5) | 36 (14.6) |
| Pyrexia | 52 (16.0) | 24 (11.0) | 13 (4.3) | 14 (5.7) |
| Lymphopenia | 36 (11.0) | 17 (7.8) | 3 (1.0) | 3 (1.2) |
| Nausea | 35 (10.7) | 23 (10.5) | 26 (8.6) | 17 (6.9) |
| Respiratory tract infection viral | 33 (10.1) | 9 (4.1) | 21 (7.0) | 10 (4.1) |
| Chills | 33 (10.1) | 11 (5.0) | 1 (0.3) | 3 (1.2) |
| Lymphocyte count decreased | 32 (9.8) | 17 (7.8) | 6 (2.0) | 4 (1.6) |
| Abdominal pain | 31 (9.5) | 12 (5.5) | 13 (4.3) | 8 (3.3) |
| Respiratory tract infection | 28 (8.6) | 14 (6. (14) | 2 (7.9) | 14 (5.7) |
| Influenza like illness | 28 (8.6) | 11 (5.0) | 5 (1.7) | 6 (2.4) |
| Back pain | 27 (8.3) | 24 (11.0) | 25 (8.3) | 28 (11.4) |
| Diarrhea | 26 (8.0) | 18 (8.2) | 32 (10.6) | 26 (10.6) |
| Hyperthermia | 25 (7.7) | 6 (2.7) | 2 (0.7) | 4 (1.6) |
| Upper respiratory tract infection | 24 (7.4) | 17 (7.8) | 21 (7.0) | 17 (6.9) |
| Pharyngitis | 22 (6.7) | 10 (4.6) | 9 (3.0) | 3 (1.2) |
| Oropharyngeal pain | 21 (6.4) | 10 (4.6) | 12 (4.0) | 7 (2.8) |
| Rhinitis | 20 (6.1) | 4 (1.8) | 14 (4.6) | 7 (2.8) |
| Toothache | 20 (6.1) | 3 (1.4) | 14 (4.6) | 7 (2.8) |
| Pain in extremity | 20 (6.1) | 11 (5.0) | 8 (2.6) | 16 (6.5) |
| Insomnia | 20 (6.1) | 13 (5.9) | 11 (3.6) | 5 (2.0) |
| Cystitis | 19 (5.8) | 6 (2.7) | 14 (4.6) | 6 (2.4) |
| Dizziness | 19 (5.8) | 5 (2.3) | 7 (2.3) | 10 (4.1) |
| Fatigue | 19 (5.8) | 9 (4.1) | 7 (2.3) | 13 (5.3) |
| Infusion related reaction | 19 (5.8) | 8 (3.7) | 2 (0.7) | 1 (0.4) |
| Anxiety | 19 (5.8) | 5 (2.3) | 11 (3.6) | 12 (4.9) |
| Asthenia | 18 (5.5) | 8 (3.7) | 18 (6.0) | 11 (4.5) |
| Dyspepsia | 17 (5.2) | 6 (2.7) | 10 (3.3) | 10 (4.1) |
| Cough | 13 (4.0) | 11 (5.0) | 8 (2.6) | 2 (0.8) |
| Sinus tachycardia | 13 (4.0) | 12 (5.5) | 8 (2.6) | 2 (0.8) |
| Urinary tract infection | 10 (3.1) | 12 (5.5) | 10 (3.3) | 19 (7.7) |
Source: Adapted from FDA Review
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.