Drug Trials Snapshots: BESPONSA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to BESPONSA Prescribing Information for complete information.
BESPONSA (inotuzumab ozogamicin)
bee SPON’sah
Pfizer Inc.
Approval date: August 17, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
BESPONSA is used to treat adults with B-cell acute lymphoblastic leukemia (ALL) whose disease has come back or has not improved after previous treatment(s).
ALL is a rapidly progressing cancer that forms in the bone marrow and results in an increased number of lymphocytes, a type of white blood cells, in the bloodstream.
How is this drug used?
BESPONSA is given by a healthcare provider directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV infusion. It takes about one hour to receive BESPONSA infusion.
BESPONSA is given once a week for the first three weeks of the treatment Cycle 1 and then according to a special schedule depending on the response.
What are the benefits of this drug?
36 percent of the 109 patients who received BESPONSA experienced no evidence of disease and full recovery of blood counts after treatment (complete remission) which lasted about 8 months. In comparison, 17 percent of the 109 patients who received other medications to treat ALL experience a complete remission that lasted about 5 months.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results established on the basis of the complete response (CR), duration of remission (DoR), and minimal residual disease (MRD) results in the initial 218 randomized patients. Results in these patients were consistent with those seen in all 326 randomized patients.
Table 2: Efficacy Results in Patients with Relapsed or Refractory B Cell Precursor ALL Who Received BESPONSA or Investigator’s Choice of Chemotherapy (FLAG, MXN/Ara C, or HIDAC)
| CRa | CRib | CR/CRia,b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BESPONSA (N=109) | HIDAC, FLAG, or MXN/Ara-C (N=109) | BESPONSA (N=109) | HIDAC, FLAG or MXN/Ara-C (N=109) | BESPONSA (N=109) | HIDAC, FLAG, or MXN/Ara-C (N=109) | ||||
| Responding (CR/CRi) patients | |||||||||
| n (%) [95% CI] | 39 (35.8) [26.8-45.5] | 19 (17.4) [10.8-25.9] | 49 (45.0) [35.4-54.8] | 13 (11.9) [6.5-19.5] | 88 (80.7) [72.1-87.7] | 32 (29.4) [21.0-38.8] | |||
| p-valuec | > | ||||||||
| DoRd | |||||||||
| n | 39 | 18 | 45 | 14 | 84 | 32 | |||
| Median, months [95% CI] | 8.0 [4.9-10.4] | 4.9 [2.9-7.2] | 4.6 [3.7-5.7] | 2.9 [0.6-5.7] | 5.4 [4.2-8.0] | 3.5 [2.9-6.6] | |||
| MRD-negativitye | |||||||||
| n | 35 | 6 | 34 | 3 | 69 | 9 | |||
| Ratef (%) [95% CI] | 35/39 (89.7) [75.8-97.1] | 6/19 (31.6) [12.6-56.6] | 34/49 (69.4) [54.6-81.7] | 3/13 (23.1) [5.0-53.8] | 69/88 (78.4) [68.4-86.5] | 9/32 (28.1) [13.7-46.7] | |||
Abbreviations: CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete hematologic recovery; DoR=duration of remission; EAC=Endpoint Adjudication Committee; FLAG=fludarabine + cytarabine + granulocyte colony-stimulating factor; HIDAC=high-dose cytarabine; HR=hazard ratio; MRD=minimal residual disease; MXN/AraC=mitoxantrone + cytarabine; N/n=number of patients; OS=overall survival; PFS=progression-free survival.
a CR, per EAC, was defined as 5% blasts="" in="" the="" bone="" marrow="" and="" the="" absence="" of="" peripheral="" blood="" leukemic="" blasts,="" full="" recovery="" of="" peripheral="" blood="" counts="" (platelets="" ≥ 100 × 109/l="" and="" absolute="" neutrophil="" counts="" [anc]≥ 1 × 109/l)="" and="" resolution="" of="" any="" extramedullary="">
b CRi, per EAC, was defined as 5% blasts="" in="" the="" bone="" marrow="" and="" the="" absence="" of="" peripheral="" blood="" leukemic="" blasts,="" incomplete="" recovery="" of="" peripheral="" blood="" counts="" (platelets=""> 100 × 109 and/or="" anc=""> 1 × 109 )="" and="" resolution="" of="" any="" extramedullary="">
c 1-sided p-value using Chi-squared test.
d DoR, based on a later cutoff date than the CR/CRi, was defined for patients who achieved CR/CRi per Investigator’s assessment as time since first response of CRa or CRib per Investigator’s assessment to the date of a PFS event or censoring date if no PFS event was documented.
e MRD-negativity was defined by flow cytometry as leukemic cells comprising 1 × 10-4> 0.01%) of="" bone="" marrow="" nucleated="">
f Rate was defined as the number of patients who achieved MRD negativity divided by the total number of patients who achieved CR/CRi per EAC.
BESPONSA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: BESPONSA worked similarly in men and women.
- Race: Most of the patients were White. Differences in how well the drug worked among races could not be determined because of the small number of patients in other races.
- Age: BESPONSA worked similarly in patients younger and older than 55 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by demographic subgroups. Because of the small sample sizes, these exploratory analyses should be interpreted with caution.
Table 3. Subgroup Analyses of Complete Response Rate by Sex, Race, Age, and Region
| BESPONSA N=109 | Investigator’s Choice N=109 | Rate Difference (97.5% CI) | |
|---|---|---|---|
| Sex | |||
| Men, n Rate Women, n Rate | 61 45 (75.4) 48 39 (81.3) | 73 17 (23.3) 36 14(38.9) | 52.1 (35.5, 68.7) 42.4 (20.2, 64.5) |
| Race | |||
| White, n Rate Black, n Rate Asian, n Rate Other, n Rate | 76 62 (81.6) 1 0 17 14 (82.4) 15 9 (60.0) | 79 23 (29.1) 2 1 (50.0) 17 3 (17.6) 11 4 (36.4) | 52.5 (37.3, 67.6) -50.0 (-100.0, 29.2) 64.7 (35.4, 94.0) 23.6 (-19.5, 66.8) |
| Age | |||
| 55 year,="" n=""> Rate (95% CI) ≥55 year, n Rate (95% CI) | 66 53 (80.3) (68.7, 89.1) 43 35 (81.4) (66.6, 91.6) | 69 22 (31.9) (21.2, 44.2) 40 10 (25.0) (12.7, 41.2) | 48.4 (31.7, 65.1) 56.4 (36.1, 76.7) |
| Region | |||
| Asia, n Rate North America, n Rate EU, n Rate Others, n Rate | 14 12 (85.7) 48 35 (72.9) 45 37 (82.2) 2 1 (50.0) | 7 2 (28.6) 52 13 (25.0) 47 15 (31.9) 3 1 (33.3) | 57.1 (13.5, 100.0) 47.9 (28.2, 67.6) 50.3 (30.4, 70.2) 16.7 (-83.3, 100.0) |
Adapted from FDA review
What are the possible side effects?
BESPONSA may cause serious and life threating liver damage. Other serious side effects include increased death rate after stem-cell transplant, bone marrow suppression, heart rhythm problems due to QT prolongation and infusion reactions.
The most common side effects of BESPONSA are low blood cell counts, infection, tiredness, bleeding, fever, nausea, headache and increase in liver tests.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize adverse reactions and laboratory abnormalities in the clinical trial. Adverse reactions included treatment-emergent all-causality events that commenced on or after Cycle 1 Day 1 within 42 days after the last dose of BESPONSA, but prior to the start of a new anticancer treatment (including HSCT).
Table 4. Adverse Reactions With ≥ 10% Incidencea in Patients With Relapsed or Refractory B Cell Precursor ALL Who Received BESPONSA or Investigator’s Choice of Chemotherapy (FLAG, MXN/Ara-C, or HIDAC)
| Body System Adverse Reaction | BESPONSA (N=164) | FLAG, MXN/Ara-C, or HIDAC (N=143b) | ||
|---|---|---|---|---|
| All Grades | ≥ Grade 3 | All Grades | ≥ Grade 3 | |
| % | % | % | % | |
| Infections | ||||
| Infectionc | 48 | 28 | 76 | 54 |
| Blood and lymphatic system disorders | ||||
| Thrombocytopeniad | 51 | 42 | 61 | 59 |
| Neutropeniae | 49 | 48 | 45 | 43 |
| Anemiaf | 36 | 24 | 59 | 47 |
| Leukopeniag | 35 | 33 | 43 | 42 |
| Febrile neutropenia | 26 | 26 | 53 | 53 |
| Lymphopeniah | 18 | 16 | 27 | 26 |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 12 | 1 | 13 | 2 |
| Nervous system disorders | ||||
| HeadacheI | 28 | 2 | 27 | 1 |
| Vascular disorders | ||||
| Hemorrhagej | 33 | 5 | 28 | 5 |
| Gastrointestinal disorders | ||||
| Nausea | 31 | 2 | 46 | 0 |
| Abdominal paink | 23 | 3 | 23 | 1 |
| Diarrhea | 17 | 1 | 38 | 1 |
| Constipation | 16 | 0 | 24 | 0 |
| Vomiting | 15 | 1 | 24 | 0 |
| Stomatitisl | 13 | 2 | 26 | 3 |
| Hepatobiliary disorders | ||||
| Hyperbilirubinemia | 21 | 5 | 17 | 6 |
| General disorders and administration site conditions | ||||
| Fatiguem | 35 | 5 | 25 | 3 |
| Pyrexia | 32 | 3 | 42 | 6 |
| Chills | 11 | 0 | 11 | 0 |
| Investigations | ||||
| Transaminases increasedn | 26 | 7 | 13 | 5 |
| Gamma-glutamyltransferase increased | 21 | 10 | 8 | 4 |
| Alkaline phosphatase increased | 13 | 2 | 7 | 0 |
Abbreviations: ALL=acute lymphoblastic leukemia; FLAG=fludarabine + cytarabine + granulocyte colony-stimulating factor; HIDAC=high dose cytarabine; HSCT=hematopoietic stem cell transplant; MXN/Ara-C=mitoxantrone + cytarabine; N=number of patients; NCI CTCAE=National Cancer Institute Common Toxicity Criteria for Adverse Events.
a Only adverse reactions with ≥ 10% incidence in the BESPONSA arm are included.
b 19 patients randomized to FLAG, MXN/Ara-C, or HIDAC did not receive treatment.
c Infection includes any reported preferred terms for BESPONSA retrieved in the System Organ Class Infections and infestations.
d Thrombocytopenia includes the following reported preferred terms: Platelet count decreased and Thrombocytopenia.
e Neutropenia includes the following reported preferred terms: Neutropenia and Neutrophil count decreased.
f Anemia includes the following reported preferred terms: Anemia and Hemoglobin decreased.
g Leukopenia includes the following reported preferred terms: Leukopenia, Monocytopenia, and White blood cell count decreased.
h Lymphopenia includes the following reported preferred terms: B-lymphocyte count decreased, Lymphocyte count decreased, and Lymphopenia.
I Headache includes the following reported preferred terms: Headache, Migraine, and Sinus headache.
j Hemorrhage includes reported preferred terms for BESPONSA retrieved in the Standard MedDRA Query (narrow) for Hemorrhage terms (excluding laboratory terms), resulting in the following preferred terms: Conjunctival hemorrhage, Contusion, Ecchymosis, Epistaxis, Eyelid bleeding, Gastrointestinal hemorrhage, Gastritis hemorrhagic, Gingival bleeding, Hematemesis, Hematochezia, Hematotympanum, Hematuria, Hemorrhage intracranial, Hemorrhage subcutaneous, Hemorrhoidal hemorrhage, Intra-abdominal hemorrhage, Lip hemorrhage, Lower gastrointestinal hemorrhage, Mesenteric hemorrhage, Metrorrhagia, Mouth hemorrhage, Muscle hemorrhage, Oral mucosa hematoma, Petechiae, Post‑procedural hematoma, Rectal hemorrhage, Shock hemorrhagic, Subcutaneous hematoma, Subdural hematoma, Upper gastrointestinal hemorrhage, and Vaginal hemorrhage.
k Abdominal pain includes the following reported preferred terms: Abdominal pain, Abdominal pain lower, Abdominal pain upper, Abdominal tenderness, Esophageal pain, and Hepatic pain.
l Stomatitis includes the following reported preferred terms: Aphthous ulcer, Mucosal inflammation, Mouth ulceration, Oral pain, Oropharyngeal pain, and Stomatitis.
m Fatigue includes the following reported preferred terms: Asthenia and Fatigue.
n Transaminases increased includes the following reported preferred terms: Aspartate aminotransferase increased, Alanine aminotransferase increased, Hepatocellular injury, and Hypertransaminasemia.
BESPONSA Prescribing Information
Table 5. Laboratory Abnormalities in Patients with Relapsed or Refractory B-Cell Precursor ALL Who Received BESPONSA or Investigator’s Choice of Chemotherapy (FLAG, MXN/Ara-C, or HIDAC)
| Laboratory Abnormalitya | N | BESPONSA | N | FLAG, MXN/Ara-C, or HIDAC | ||
|---|---|---|---|---|---|---|
| All Grades | Grade 3/4 | All Grades | Grade 3/4 | |||
| % | % | % | % | |||
| Hematology | ||||||
| Platelet count decreased | 161 | 98 | 76 | 142 | 100 | 99 |
| Hemoglobin decreased | 161 | 94 | 40 | 142 | 100 | 70 |
| Leukocytes decreased | 161 | 95 | 82 | 142 | 99 | 98 |
| Neutrophil count decreased | 160 | 94 | 86 | 130 | 93 | 88 |
| Lymphocytes (absolute) decreased | 160 | 93 | 71 | 127 | 97 | 91 |
| Chemistry | ||||||
| GGT increased | 148 | 67 | 18 | 111 | 68 | 17 |
| AST increased | 160 | 71 | 4 | 134 | 38 | 4 |
| ALP increased | 158 | 57 | 1 | 133 | 52 | 3 |
| ALT increased | 161 | 49 | 4 | 137 | 46 | 4 |
| Blood bilirubin increased | 161 | 36 | 5 | 138 | 35 | 6 |
| Lipase increased | 139 | 32 | 13 | 90 | 20 | 2 |
| Hyperuricemia | 158 | 16 | 3 | 122 | 11 | 0 |
| Amylase increased | 143 | 15 | 2 | 102 | 9 | 1 |
Abbreviations: ALL=acute lymphoblastic leukemia; ALP=alkaline phosphatase; ALT=alanine aminotransferase; AST=aspartate aminotransferase; FLAG=fludarabine + cytarabine + granulocyte colony-stimulating factor; GGT=gamma-glutamyltransferase; HIDAC=high dose cytarabine; MXN/Ara-C=mitoxantrone + cytarabine; N=number of patients; NCI CTCAE=National Cancer Institute Common Toxicity Criteria for Adverse Events.
a Laboratory abnormalities were summarized up to the end of treatment + 42 days but prior to the start of a new anti-cancer therapy.
BESPONSA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: Most of the patients were White. Differences in the occurrence of side effects among races could not be determined because of the small number of patients in other races.
- Age: The occurrence of side effects was similar in patients younger and older than 55 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table below summarizes hepatotoxicity events during the clinical trials by sex, age and race subgroup. Because of the small sample sizes, these exploratory analyses should be interpreted with caution.
Table 6. Subgroup Analyses of Hepatotoxicity Adverse Events
| Demographic Subgroup | BESPONSA N=164 | Investigator Choice N=143 | Relative Risk (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Total, N | n (%) | Total, N | ||
| Overall | 43 (26.2) | 164 | 25 (17.5) | 143 | 1.50 (0.97, 2.33) |
| SEX | |||||
| Men | 31 (34.1) | 91 | 17 (18.5) | 92 | 1.84 (1.10, 3.09) |
| Women | 12 (16.4) | 73 | 8 (15.7) | 51 | 1.05 (0.46, 2.38) |
| AGE | |||||
| 55=""> | 26 (25.0) | 104 | 17 (18.7) | 91 | 1.34 (0.78, 2.30) |
| >= 55 years | 17 (28.3) | 60 | 8 (15.4) | 52 | 1.84 (0.87, 3.91) |
| RACE | |||||
| White | 31 (27.7) | 112 | 19 (18.1) | 105 | 1.53 (0.92, 2.54) |
| Black or African American | 2 (50.0) | 4 | 1 (33.3) | 3 | 1.50 (0.23, 9.80) |
| Asian | 6 (19.4) | 31 | 2 (10.0) | 20 | 1.94 (0.43, 8.66) |
| American Indian | 0 (0.0) | 0 | 0 (0.0) | 0 | -- |
| Native Hawaiian | 0 (0.0) | 0 | 0 (0.0) | 0 | -- |
| Other | 4 (23.5) | 17 | 3 (20.0) | 15 | 1.18 (0.31, 4.43) |
| Missing Race | 0 (0.0) | 0 | 0 (0.0) | 0 | -- |
| REGION | |||||
| Asia | 5 (19.2) | 26 | 2 (13.3) | 15 | 1.44 (0.32, 6.54) |
| EU | 20 (32.8) | 61 | 9 (16.7) | 54 | 1.97 (0.98, 3.95) |
| North America | 17 (22.7) | 75 | 14 (18.9) | 74 | 1.20 (0.64, 2.25) |
| Others | 1 (50.0) | 2 | 0 (0.0) | 0 | -- |
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved BESPONSA based on evidence from one clinical trial (INO-VATE ALL [NCT01564784]) that enrolled 326 patients with ALL whose disease has come back or has not improved after previous treatment(s).
The trial was conducted in United States, Canada, European Union, Japan, Korea, Singapore, Taiwan and Australia.
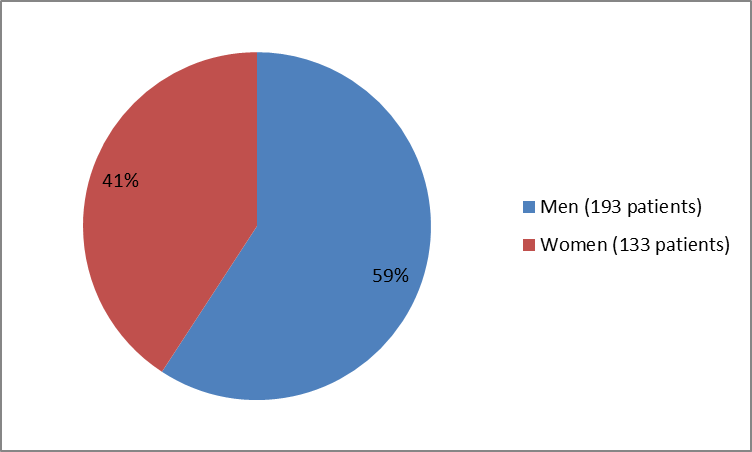
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
FDA review
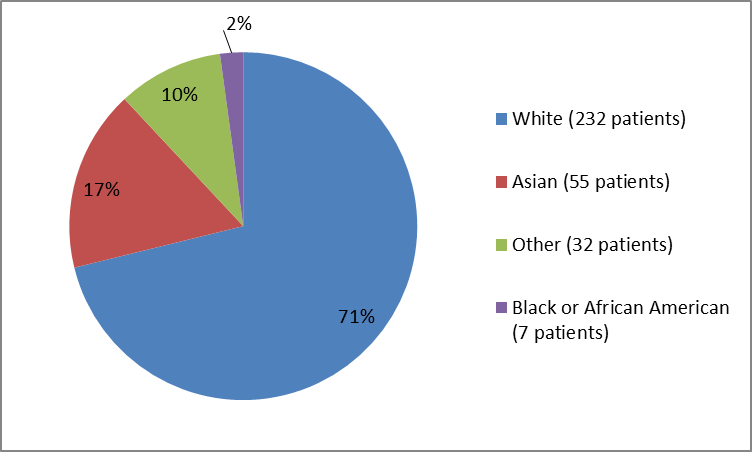
Figure 2. Baseline Demographics by Race
FDA review
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 232 | 71 |
| Black or African American | 7 | 2 |
| Asian | 55 | 17 |
| Other | 32 | 10 |
FDA review
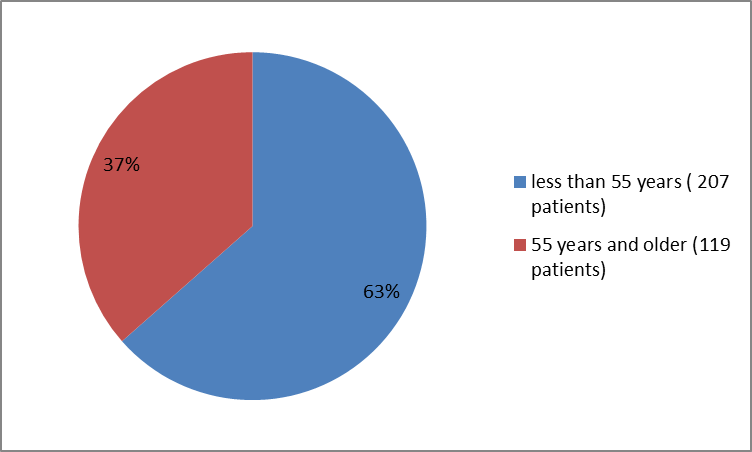
Figure 3 summarizes how many patients of certain age were enrolled in the clinical trial.
Figure 3. Baseline Demographics by Age
FDA review
Who participated in the trials?
The table below summarizes demographics of all patients enrolled in the clinical trial.
Table 7. Baseline Demographics of Patients Enrolled in the Clinical Trial
| Demographic Characteristic | BESPONSA N=164 | Active Comparator (Investigator’s Choice) N=162 | Total N=326 |
|---|---|---|---|
| Sex Men Women | 91 (55.5) 73 (44.5) | 102 (63.0) 60 (37.0) | 193 (59) 133 (41) |
| Race White Black or African American Asian Other | 112 (68.3) 4 (2.4) 31 (18.9) 17 (10.4) | 120 (74.1) 3 (1.9) 24 (14.8) 15 (9.3) | 232 (71) 7 (2) 55 (17) 32 (10) |
| Age 55 years=""> ≥55 years | 104 (63.4) 60 (36.6) | 103 (63.6) 59 (36.4) | 207 (63) 119 (37) |
FDA review
Table 8 . Baseline Demographics of Patients (ITT population)
| Demographic Characteristic | BESPONSA N=109 | Active Comparator (Investigator’s Choice) N=109 |
|---|---|---|
| Sex, n (%) Men Women | 61 48 | 73 36 |
| Race, n (%) White Black or African American Asian Other | 76 (69.7) 1 (0.9) 17 (15.6) 15 (13.8) | 79 (72.5) 2 (1.8) 17 (15.6) 11 (10.1) |
| Age, n (%) 55 years=""> ≥55 years ≥65 years | 66 (60.6) 43 (39.4) 23 (21.1) | 69 (63.3) 40 (36.7) 14 (12.8) |
FDA review
How were the trials designed?
There was one trial that evaluated the benefit and side effects of BESPONSA in patients with ALL whose disease has come back or has not improved after previous treatment(s). All patients had a certain type of ALL called B cell precursor ALL.
Patients received either BESPONSA infusion once a day or some other type of treatment for ALL per investigator’s choice. Both, patients and investigators knew which treatment has been given.
The benefit of BESPONSA was evaluated in the first 218 patients by measuring
- how many patients reached complete remission (no evidence of disease) with full or partial recovery of blood counts after treatment,
- how long those patients remained in remission, and
- how many patients did not show signs of minimal disease in the bone marrow.
How were the trials designed?
The safety and efficacy of BESPONSA were established in one active-controlled, randomized (1:1), open label, multicenter trial in patients with relapsed or refractory ALL. Patients were ≥ 18 years of age with Philadelphia chromosome-negative or Philadelphia chromosome-positive relapsed or refractory B cell precursor ALL.
Patients received BESPONSA or Investigator’s choice of chemotherapy (fludarabine + cytarabine + granulocyte colony-stimulating factor [FLAG], mitoxantrone + cytarabine [MXN/Ara-C], or high dose cytarabine [HIDAC]) until disease progression or unacceptable toxicity.
The efficacy was established on the basis of the rate of CR, the duration of CR, and proportion of MRD-negative CR ( 1="" x="" 10-4="" of="" bone="" marrow="" nucleated="" cells="" by="" flow="" cytometry)="" in="" the="" first="" 218="" patients="">
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.