Drug Trials Snapshots: ACCRUFER
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the ACCRUFER Package Insert for complete information.
ACCRUFER (ferric maltol)
ak-roo-fer
Shield Therapeutics
Approval date: July 25, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ACCRUFER is a drug used for the treatment of adults with low iron stores in the body.
How is this drug used?
ACCRUFER is a capsule taken two times a day on an empty stomach.
What are the benefits of this drug?
ACCRUFER was better than placebo in restoring iron stores in the body.
What are the benefits of this drug (results of trials used to assess efficacy)?
The tables below present efficacy results from the trials based on the mean difference in hemoglobin (Hb) concentration from baseline to the last week of treatment.
Table 2. Patients with Inflammatory Bowel Disease (IBD)-Summary of Hemoglobin Concentration (g/dL) and Change from Baseline to Week 12 in Trials 1 and 2 - Analysis Using Multiple Imputation - Full Analysis Set Population
| Visit (Week) Statistic | ACCRUFER (N = 64) | Placebo (N =64) | |
|---|---|---|---|
| Treatment Comparison | Difference in Change from Baseline | ||
| LSM Difference (SE) ACCRUFER – Placebo) | 1-sided lower 97.5% CI | p-value | |
| Baseline | |||
| Mean (SD) | 11.0 (1.03) | 11.10 (0.85) | |
| Mean change from baseline to Week 12 | |||
| LS Mean (SE) | 2.25 (0.12) | 0.06 (0.13) | |
| ACCRUFER versus placebo | 2.18 (0.19) | (1.81) | <0.0001 |
Note: Multiple imputation was based on treatment, sex, disease [UC or CD], and Hb concentration at baseline, Week 4, and 8. For each imputed dataset, the change from baseline to Week 12 was analyzed using an ANCOVA model with treatment as the factor and sex, disease, baseline Hb concentration as covariates. SD=standard deviation; LS=Least Squares; SE-standard error
Prescribing Information
Table 3. Patients with Chronic Kidney Disease- Summary of Hemoglobin Concentration (g/dL) and Change from Baseline to Week 16 in Trial 3- Analysis Using Multiple Imputation – Intent-to-Treat Population
| Visit (Week) Statistic | ACCRUFER (N = 111) | Placebo (N = 56) | |
|---|---|---|---|
| Treatment Comparison | Difference in Change from Baseline | ||
| LSM Difference (SE) ACCRUFER – Placebo | 95% CI | p-value | |
| Baseline | |||
| Mean (SD) | 10.06 (0.77) | 10.03 (0.82) | |
| Mean change from baseline to Week 16 | |||
| LS Mean (SE) | 0.50 (0.12) | -0.02 (0.16) | |
| ACCRUFER versus placebo | 0.52 (0.21) | (0.10, 0.93) | 0.0149 |
Note: Multiple imputation was based on treatment, sex, eGFR at baseline, and Hb concentration at baseline, Week 4 and 8. For each imputed dataset, the change from baseline to Week 16 was analyzed using an ANCOVA model with treatment as the factor and baseline Hb concentration, baseline eGFR as covariates SD=standard deviation; LS=Least Squares; SE-standard error
Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: ACCRUFER was similarly effective in men and women.
- Race: The majority of patients were White. The number of patients in other races was limited. Therefore, differences among races in how well the drug worked could not be determined.
- Age: ACCRUFER was similarly effective in patients below and above 60 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Because of predominantly White population younger than 65 years, only sex differences were analyzed in combined Trials 1 and 2 efficacy population.
Table 4. Summary of Subgroup Analyses by Sex, Race and Age in Trials 1 and 2 - FAS
| LS Mean Difference (SE) | 1-sided 97.5% CI | |
|---|---|---|
| Sex | ||
| Primary Analysis (n=128 [100%]) | 2.18 (0.19) | (1.81) |
| Women (n=83 [64.8%]) | 1.95 (0.19) | (1.57) |
| Men (n=45 [35.2%]) | 2.42 (0.30) | (1.82) |
LS=Least Squares; SE-standard error; CI=confidence intervals
FDA Review
Subpopulation efficacy analyses for sex, race and age in population from Trial 3 are presented below.
Table 5. Summary of Subgroup Analyses by Sex, Race and Age in Trial 3 - ITT
| LS Mean Difference (SE) | 95% CI | |
|---|---|---|
| Sex | ||
| Race | ||
| Age group (years) | ||
| Primary Analysis (n=167 [100%]) | 0.52 (0.21) | (0.10, 0.93) |
| Women (n=117 [70.1%]) | 0.53 (0.21) | (0.12, 0.94) |
| Male (n=50 [29.9%]) | 0.34 (0.50) | (-0.64, 1.32) |
| White (n=123 [73.7%]) | 0.52 (0.24) | (0.05, 0.99) |
| All other (n=44 [26.3%]) | 0.33 (0.45) | (-0.57, 1.23) |
| <65 (n=53 [31.7%]) | 0.34 (0.40) | (-0.43, 1.12) |
| ≥65 (n=114 [68.3%]) | 0.53 (0.27) | (0, 1.06) |
LS=Least Squares; SE-standard error; CI=confidence intervals
FDA Review
What are the possible side effects?
ACCRUFER can cause serious side effects including increased risk of inflammatory bowel disease flare and iron overload in the body.
The most common side effects of ACCRUFER are gas, diarrhea, constipation, stool color change, nausea, vomiting, and abdominal discomfort, bloating and pain.
What are the possible side effects (results of trials used to assess safety)?
The table below presents all adverse reactions occurring in the placebo-controlled period of the pooled randomized trials occurring at a rate of > 1% in the treated group, and for which the rate for ACCRUFER exceeds the rate for placebo.
Table 6. Adverse Reactions Reported by ≥1% of Patients Treated with ACCRUFER during Placebo Controlled Period of Pooled Trials (1, 2 and 3)
| Body System Adverse Reaction | ACCRUFER (N = 175) | Placebo (N = 120) |
|---|---|---|
| Gastrointestinal | ||
| Flatulence | 4.6% | 0% |
| Diarrhea | 4% | 1.7% |
| Constipation | 4% | 0.8% |
| Feces discolored | 4% | 0.8% |
| Abdominal pain | 2.9% | 2.5% |
| Nausea | 1.7% | 0.8% |
| Vomiting | 1.7% | 0% |
| Abdominal Discomfort | 1.1% | 0% |
| Abdominal Distension | 1.1% | 0% |
Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients were White. The number of patients in other races was limited. Therefore, differences in the occurrence of side effects could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 60 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Subgroup analyses of adverse events are presented in Tables 7-9.
Table 7. Adverse Events by Sex Subgroup
| Adverse Events | ACCRUFER (N=175) | Placebo (N=120) | ||
|---|---|---|---|---|
| Men (n=57) | Women (n=118) | Men (n=38) | Women (n=82) | |
| Any TEAE, x (%) | 35 (61.4) | 79 (66.9) | 27 (71) | 60 (73.2) |
| SAE, x (%) | 11 (19.3) | 13 (11.0) | 4 (10.5) | 10 (12.2) |
Table 8. Adverse Events by Race Subgroup
| Adverse Events | ACCRUFER (N=175) | Placebo (N=120) | ||
|---|---|---|---|---|
| White (n=143) | All Other (n=32) | White (n=102) | All Other (n=18) | |
| Any TEAE, x (%) | 93 (65) | 21 (65.6) | 77 (75.5) | 10 (55.5) |
| SAE, x (%) | 19 (13.3) | 5 (15.6) | 12 (11.8) | 2 (11.1) |
Table 9. Adverse Events by Age Subgroup
| Adverse Events | ACCRUFER (N=175) | Placebo (N=120) | |||
|---|---|---|---|---|---|
| <60 years (n=85) | ≥60 years (n=90) | <60 years (n=74) | ≥60 years (n=46) | ||
| Any TEAE, x (%) | 56 (65.9) | 58 (64.4) | 53 (71.6) | 34 (73.9) | |
| SAE, x (%) | 6 (7) | 18 (20) | 4 (5.4) | 10 (21.7) | |
TEAE=treatment -emergent adverse events; SAE=serious adverse events
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved ACCRUFER based on evidence from three clinical trials (Trial 1/ NCT01252221, Trial 2/NCT01340872 and Trial 3/NCT02968368). All 295 patients had low iron stores in the body and consequent iron deficiency anemia. In the first two trials low iron was caused by patients’ inflammatory bowel disease (IBD) and in the last trial, by long standing (chronic) kidney disease or CKD.
Trials were conducted at 79 sites in Europe and the United States.
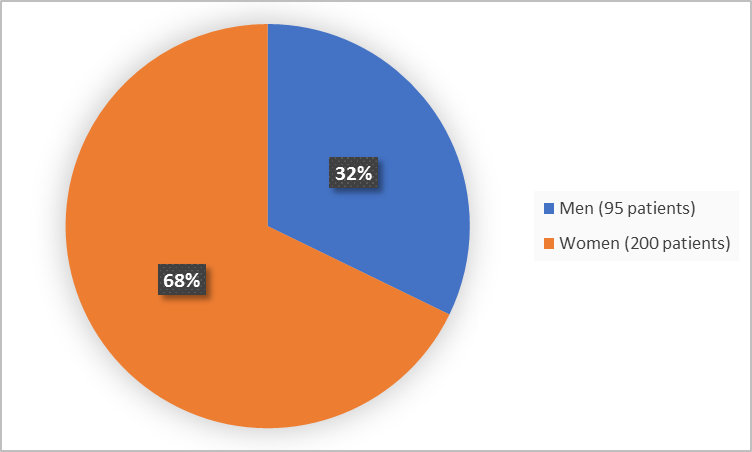
Figure 1 summarizes how many men and women were in the combined clinical trials.
Figure 1. Demographics by Sex (safety population)
FDA Review
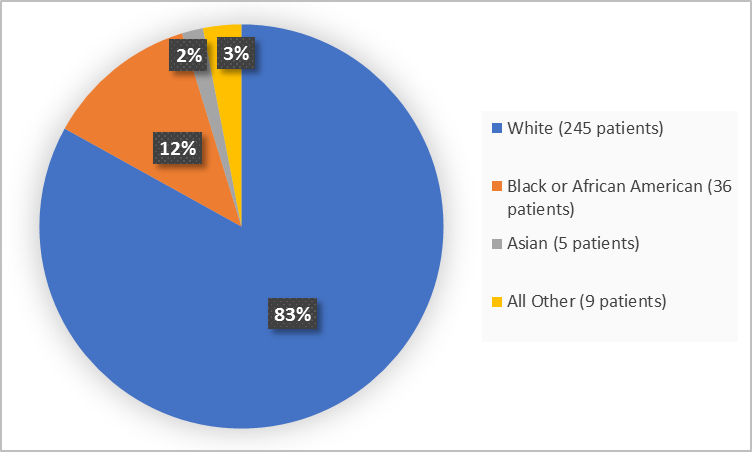
Figure 2 summarizes the percentage of patients by race in the combined clinical trials.
Figure 2. Demographics by Race (safety population)
FDA Review
Table 1. Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 245 | 83 |
| Black or African American | 36 | 12 |
| Asian | 5 | 2 |
| All Other | 9 | 3 |
FDA Review
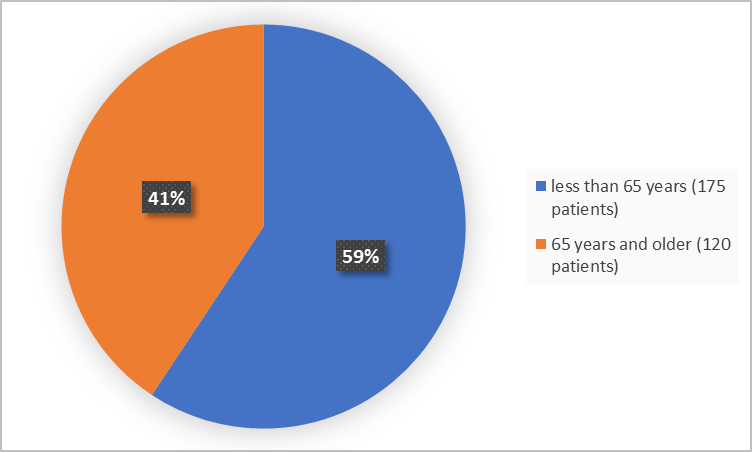
Figure 3 summarizes the percentage of patients by age group in the combined clinical trial.
Figure 3. Demographics by Age (safety population)
Clinical Trial Data
Who participated in the trials?
The FDA approved ACCRUFER based on evidence from three clinical trials. Combined demographics representing the safety population are presented below.
Table 10. Trial Demographics (safety population-Trials 1,2 and 3 combined)
| Demographic Parameter | ACCRUFER (N=175) n (%) | Placebo (N=120) n (%) | Total (N=295) n (%) | |
|---|---|---|---|---|
| Sex | ||||
| Race | ||||
| Age | ||||
| Age Group | ||||
| Ethnicity | ||||
| Country | ||||
| Women | 118 (67.4) | 82 (68.3) | 200 (98.3) | |
| Men | 57 (32.6) | 38 (31.7) | 95 (32.2) | |
| White | 143 (81.7) | 102 (85) | 245 (83) | |
| Black or African American | 23 (13.1) | 13 (10.8) | 36 (12.2) | |
| Asian | 3 (1.7) | 2 (1.7) | 5 (1.7) | |
| American Indian or Alaska Native | 1 (0.6) | 0 | 1 (0.3) | |
| Other | 5 (2.9) | 3 (2.5) | 8 (2.7) | |
| Mean (SD) | 58.1 (18.8) | 51 (18.3) | 55.2 (18.8) | |
| Median | 61 | 49 | 57 | |
| Range (Min-Max) | 18-90 | 19-83 | 18-90 | |
| < 65 years | 95 (54.3) | 80 (66.7) | 175(59.3) | |
| ≥ 65 years | 80 (45.7) | 40 (33.3) | 120 (40.6) | |
| ≥ 75 years | 43 (24.6) | 26 (21.7) | 59 (20) | |
| Hispanic or Latino | 26 (14.9) | 14 (11.7) | 40 (13.6) | |
| Not Hispanic or Latino | 145 (82.9) | 105 (87.5) | 250 (84.7) | |
| Not Reported | 4 (2.3) | 1 (0.8) | 5 (1.7) | |
| Germany | 34 (19.4) | 33 (25.0) | 67 (22.7) | |
| Hungary | 11 (6.3) | 15 (12.5) | 26 (8.8) | |
| Austria | 4 (2.3) | 6 (5.0) | 10 (3.4) | |
| UK | 15 (8.6) | 10 (8.3) | 25(8.5) | |
| US | 111 (63.4) | 56 (46.7) | 167 (59.6) | |
Adapted from FDA Review
How were the trials designed?
ACCRUFER was evaluated in three clinical trials of 295 patients with low iron stores in the body and iron deficiency anemia as confirmed by blood tests (measuring hemoglobin and ferritin).
In Trials 1 and 2 all patients also had IBS which often causes low iron stores in the body. Patients were randomly assigned to receive either ACCRUFER twice daily or a matched placebo control for 12 weeks. Neither the patients nor the investigators knew which treatment was given until the end of 12 weeks. After 12 weeks, patients from both groups could continue taking ACCRUFER for additional 52 weeks.
In Trial 3 all patients also had CKD which often causes low iron stores in the body. Patients were randomly assigned to receive either ACCRUFER twice daily or a matched placebo control for 16 weeks. Neither the patients nor the investigators knew which treatment was given until the end of 16 weeks.
The benefit of ACCRUFER was assessed by measuring improvement in hemoglobin and iron stores at the end of the treatment and comparing it to placebo.
How were the trials designed?
There were three trials that provided data for the safety and efficacy of ACCRUFER for the treatment of iron deficiency anemia.
Two randomized, placebo-controlled trials (Trials 1 and 2) enrolled patients 18-76 years old with quiescent IBD and baseline hemoglobin (Hb) concentrations between 9.5 g/dL and 12 /13 g/dL for women / men and ferritin < 30 μg/L. All patients had discontinued prior oral ferrous product treatment due to lack of efficacy or inability to tolerate oral iron replacement products. Patients were randomized 1:1 to receive either 30 mg ACCRUFER twice daily or a matched placebo control for 12 weeks. Following completion of the 12-week placebo-controlled phase of the studies, eligible patients transitioned to ACCRUFER 30 mg twice daily open-label treatment for an additional 52 weeks.
Trial 3, a randomized, placebo-controlled trial, enrolled patients 30-90 years old with non-dialysis dependent chronic kidney disease (CKD) and baseline Hb concentrations between 8 g/dL and 11 g/dL and ferritin < 25 μg/L with a transferrin saturation (TSAT) <25% or ferritin < 50 μg/L with a TSAT <15%. Patients were randomized 2:1 to receive either 30 mg ACCRUFER twice daily or a matched placebo control for 16 weeks.
The major efficacy outcome was the mean difference in Hb concentration from baseline to the end of respective 12- or 16-week treatment between ACCRUFER and placebo.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.