Drug Trials Snapshot: Tresiba
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the TRESIBA Prescribing Information for complete information.
TRESIBA (insulin degludec injection)
tre-SI-bah
Novo Nordisk
Approval date: September 25, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TRESIBA is a long-acting insulin that improves blood sugar control in adults with diabetes mellitus (DM). It can be used in adult patients with type 1 or type 2 DM.
How is this drug used?
TRESIBA is available as a liquid that comes in a prefilled pen. It is injected once daily under the skin (subcutaneously).
What are the benefits of this drug?
In patients with type 1 and type 2 diabetes who need better blood sugar control, treatment with TRESIBA can lower HbA1c (hemoglobin A1c, which is a measure of blood sugar control). TRESIBA’s ability to lower HbA1c is in line with other, long-acting insulin products on the market.
What are the benefits of this drug (results of trials used to assess efficacy)?
The efficacy of TRESIBA in patients with type 1 DM was evaluated in three trials (referred to here as Trials A, B, and C). The efficacy of TRESIBA in patients with type 2 DM was evaluated in six trials (Trials D through I). Most of the studies were a non-inferiority design which is a design to demonstrate that TRESIBA works no worse than the comparator (i.e., provides an average change in HbA1c no smaller than 0.4% less than the comparator insulin product). Study I was a superiority study (i.e. designed to show that TRESIBA was better than an oral drug comparator). Table 3 below summarizes the results for the primary efficacy endpoint, the change from the start of the trials (baseline) in HbA1c to week 26 (trials B, C, E, F, and I) and week 52 (trials A, D, and H) for TRESIBA minus the comparator for each trial.
Table 3. Change from baseline in HbA1c (%) by trial
| Study | Primary Hypothesis |
Treatment Groups | Treatment Difference (TRESIBA — Control) |
|
|---|---|---|---|---|
| LS Mean | 95% CI | |||
| Type 1 Diabetes Mellitus (T1DM) | ||||
| 3583 (A) | Non-inferiority | TRESIBA (IDeg 100) IGlar |
-0.01 | (-0.14, 0.12) |
| 3585 (B) | Non-inferiority | TRESIBA (IDeg 100) IDet |
-0.08 | (-0.23, 0.06) |
| 3770 (C) | Non-inferiority | TRESIBA (IDeg 100 Alt TRESIBA (IDeg 100) IGlar |
+0.17 +0.17 |
(0.04, 0.31) (0.04, 0.30) |
| Type 2 Diabetes Mellitus (T2DM) | ||||
| 3579 (D) | Non-inferiority | TRESIBA (IDeg 100) IGlar |
+0.08 | (-0.05, 0.21) |
| 3672 (E) | Non-inferiority | TRESIBA (IDeg 200) IGlar |
+0.05 | (-0.11, 0.20) |
| 3586 (F) | Non-inferiority | TRESIBA (IDeg 100) IGlar |
+0.08 | (-0.05, 0.22) |
| 3668 (G) | Non-inferiority | TRESIBA (IDeg 100 Flex) TRESIBA (IDeg 100) IGlar |
+0.04 +0.18 |
(-0.12, 0.19) (0.02, 0.33) |
| 3582 (H) | Non-inferiority | TRESIBA (IDeg 100) IGlar |
+0.07 | (-0.06, 0.20) |
| 3580 (I) | Superiority | TRESIBA (IDeg 100 Alt) Sitagliptin |
-0.44 | (-0.62, -0.25) |
IDeg 100 – insulin degludec 100 U/mL; IDeg 100 Alt – insulin degludec 100 U/mL at alternating times; IDeg 200 – insulin degludec 200 U/mL; IGlar - insulin glargine 100 U/mL; IDet - insulin detemir 100 U/mL; IAsp- NovoRapid/NovoLog 100 U/mL.
FDA Statistical Review
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: TRESIBA worked similarly in men and women.
- Race: In patients with type 1 DM, TRESIBA worked similarly in Whites and Asians. There were too few patients in other racial groups with type 1 DM to determine whether they responded differently to TRESIBA. In patients with type 2 DM, TRESIBA worked similarly among all racial groups studied.
- Age: TRESIBA worked similarly in patients below and above 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Figures 7 (for type 1 DM) and 8 (for type 2 DM) below summarizes the results for the primary efficacy endpoint, the change in HbA1c from the start of each trial to week 26 (trials B, C, E, F, and I) and 52 (trials A, D, and H) for TRESIBA minus the comparator by sex, age, race, and ethnicity. Trials with the same comparator are combined to allow for the largest possible sample sizes in each subgroup, and since comparisons of the treatment effect across subgroups were consistent across trials.
Figure 7. Difference (95% Confidence Interval) in Average Change in HbA1c (%) from the Start of Each Trial in T1DM
IGlar - insulin glargine 100 U/mL; T1DM – Type 1 diabetes mellitus; T2DM – Type 2 diabetes mellitus;
P-value for statistical test measuring whether the treatment effect differs across subgroups (i.e., p-value for test of treatment-by-subgroup interaction) for combined analysis of studies A and C, and combined analysis of studies D through H, respectively: Sex: 0.13 and 0.60; Age: 0.72 and 0.52; Race: NA, and 0.72; Ethnicity: 0.58, and 0.75
Figure 8. Difference (95% Confidence Interval) in Average Change in HbA1c (%) from the Start of Each Trial in T2DM
IGlar - insulin glargine 100 U/mL; T1DM – Type 1 diabetes mellitus; T2DM – Type 2 diabetes mellitus;
P-value for statistical test measuring whether the treatment effect differs across subgroups (i.e., p-value for test of treatment-by-subgroup interaction) for combined analysis of studies A and C, and combined analysis of studies D through H, respectively: Sex: 0.13, and 0.79; Age: 0.72, and 0.65; Race: NA, and 0.63; Ethnicity: 0.58, and 0.93
What are the possible side effects?
The most common side effects were low blood sugar (hypoglycemia), allergic reactions, injection site reactions, pitting at the injection site (lipodystrophy), itching, rash, swelling, and weight gain.
What are the possible side effects (results of trials used to assess safety)?
The tables below summarize common adverse reactions for the two trial populations. These reflect the Safety population, which includes any patient who received at least one dose of TRESIBA.
Table 9. Adverse Reactions Occurring in ≥5% of TRESIBA-Treated Patients with Type 1 DM
| Adverse Reaction | TRESIBA (n=1102) |
|---|---|
| Nasopharyngitis | 23.9 % |
| Upper respiratory tract infection | 11.9 % |
| Headache | 11.8 % |
| Sinusitis | 5.1 % |
| Gastroenteritis | 5.1 % |
Clinical Trial Data
Table 10. Adverse Reactions Occurring in ≥5% of TRESIBA-Treated Patients with Type 2 DM
| Adverse Reaction | TRESIBA (n=2713) |
|---|---|
| Nasopharyngitis | 12.9 % |
| Headache | 8.8 % |
| Upper respiratory tract infection | 8.4 % |
| Diarrhea | 6.3 % |
Clinical Trial Data
Were there any differences in side effects among sex, race and age?
Subgroup analyses were conducted for sex, race and age.
- Sex: The risk of side effects was similar men and women.
- Race: The majority of patients were White and Asian. The risk of side effects was similar in Whites and Asians. Differences in side effects among other races could not be determined.
- Age: The risk of side effects was similar in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize overall adverse events during the clinical trials, by subgroup. Table 11 is for the type 1 DM population and Table 12 is for the type 2 DM population.
Table 11. Overall TEAEs by Subgroup—3 Pooled Clinical Trials for the Type 1 DM Population
Demographic Parameters |
TRESIBA n/N (%) |
Control n/N (%) |
|---|---|---|
| Overall TEAEs | ||
| Sex | ||
| Men | 452/623 (72.6) | 193/261 (73.9) |
| Women | 400/479 (83.5) | 163/206 (79.1) |
| Age Group | ||
| Below 65 years | 789/1025 (77.0) | 335/438 (76.5) |
| 65 years and above | 63/77 (81.8) | 21/29 (72.4) |
| Race | ||
| White | 684/888 (77.0) | 275/374 (73.5) |
| Black or African American | 9/19 (47.4) | 4/4 (100) |
| Asian | 139/172 (80.8) | 73/85 (85.9) |
| Other | 20/23 (87.0) | 4/4 (100) |
| Ethnicity | ||
| Hispanic or Latino | 34/46 (73.9) | 14/23 (60.9) |
| Not Hispanic or Latino | 818/1056 (77.5) | 342/444 (77.0) |
Clinical Trial Data
TEAE=treatment-emergent adverse event
Percentages are calculated based on the number of subjects in the subgroup per arm.
Table 12. Overall TEAEs by Subgroup—4 Pooled Clinical Trials for the Type 2 DM Population
| Demographic Parameters | TRESIBA n/N (%) |
Control n/N (%) |
|---|---|---|
| Overall TEAES | ||
| Sex | ||
| Men | 1050/1548 (67.8) | 487/735 (66.3) |
| Women | 837/1165 (71.8) | 417/604 (69.0) |
| Age Group | ||
| Below 65 years | 1406/2047 (68.7) | 705/1039 (67.9) |
| 65 years and above | 481/666 (72.2) | 199/300 (66.3) |
| Race | ||
| White | 1369/1922 (71.2) | 620/912 (68.0) |
| Black or African American | 130/182 (71.4) | 65/98 (66.3) |
| Asian | 348/552 (63.0) | 194/296 (65.5) |
| Other | 40/57 (70.2) | 25/33 (75.8) |
| Ethnicity | ||
| Hispanic or Latino | 227/339 (67.0) | 115/178 (64.6) |
| Not Hispanic or Latino | 1632/2336 (69.9) | 773/1138 (67.9) |
| Not applicable | 28/38 (73.7) | 16/23 (69.6) |
Clinical Trial Data
TEAE=treatment-emergent adverse event
Not applicable=as defined by the company
Percentages are calculated based on the number of subjects in the subgroup per arm.
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved TRESIBA based on evidence from 3 clinical trials of 1577 (total) patients with type 1 DM and 6 clinical trials in 4048 (total) patients with type 2 DM.
The figures below summarize how many men and women participated in the clinical trials used to evaluate efficacy. Figure 1 includes the trials of patients with type 1 DM, and Figure 2 includes the trials of patients with type 2 DM.
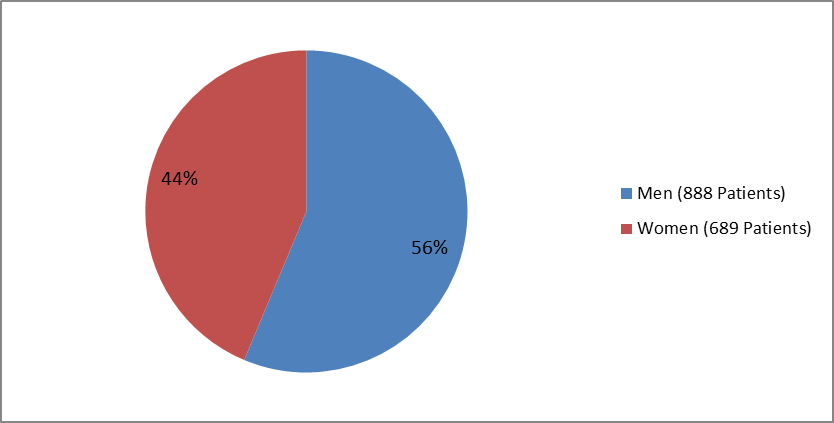
Figure 1. Patients with Type 1 DM by Sex (Total Patients=1577)
Clinical Trial Data
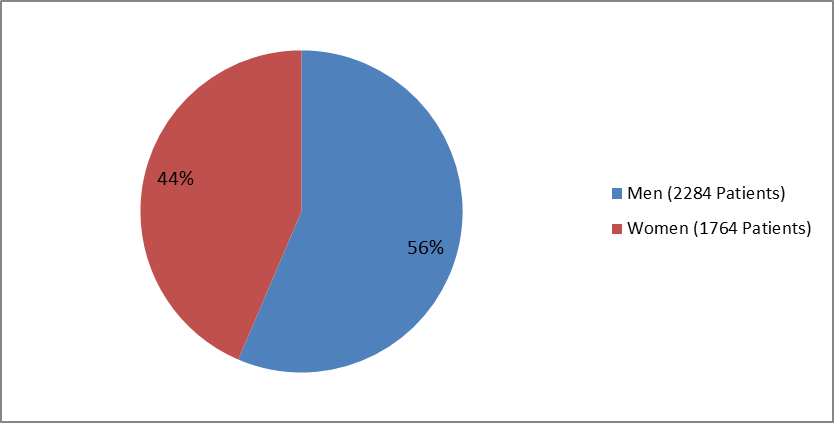
Figure 2. Patients with Type 2 DM by Sex (Total Patients=4048)
Clinical Trial Data
The figures below summarize how many patients by racial group participated in the clinical trials used to evaluate efficacy. Figure 3 includes the trials of patients with type 1 DM, and Figure 4 include the trials of patients with type 2 DM.
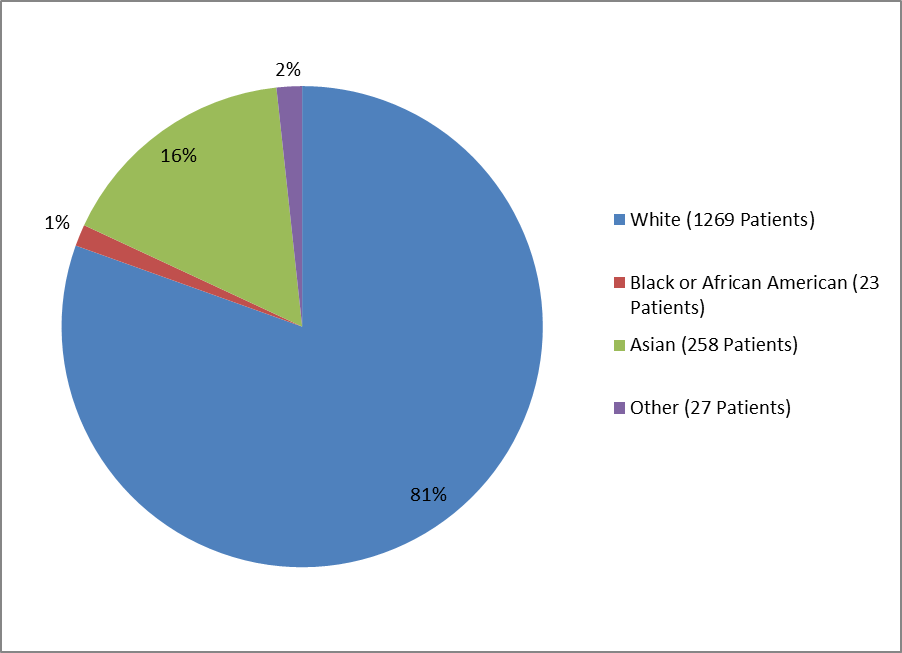
Figure 3. Patients with Type 1 DM by Racial Group
Clinical Trial Data
Table 1. Demographics of Efficacy Trials by Race—Patients with Type 1 DM
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 1269 | 81% |
| Black or African American | 23 | 1% |
| Asian | 258 | 16% |
| Other | 27 | 2% |
Clinical Trial Data
Figure 4. Patients with Type 2 DM by Racial Group
Clinical Trial Data
Table 2. Demographics of Efficacy Trials by Race—Patients with Type 2 DM
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 2823 | 70% |
| Black or African American | 281 | 7% |
| Asian | 854 | 21% |
| Other | 90 | 2% |
Clinical Trial Data
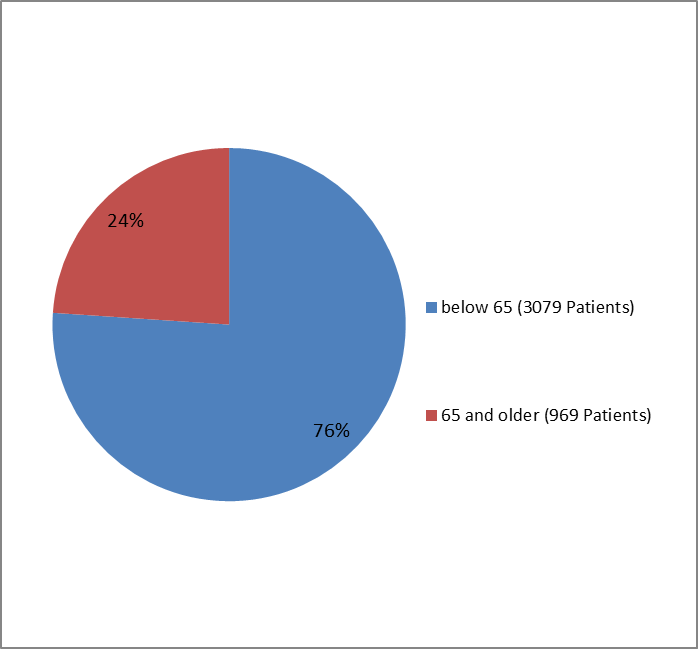
The figures below summarize how many patients by age group (at the start of the trials) participated in the clinical trials for type 1 patients (Figure 5) and type 2 patients (Figure 6).
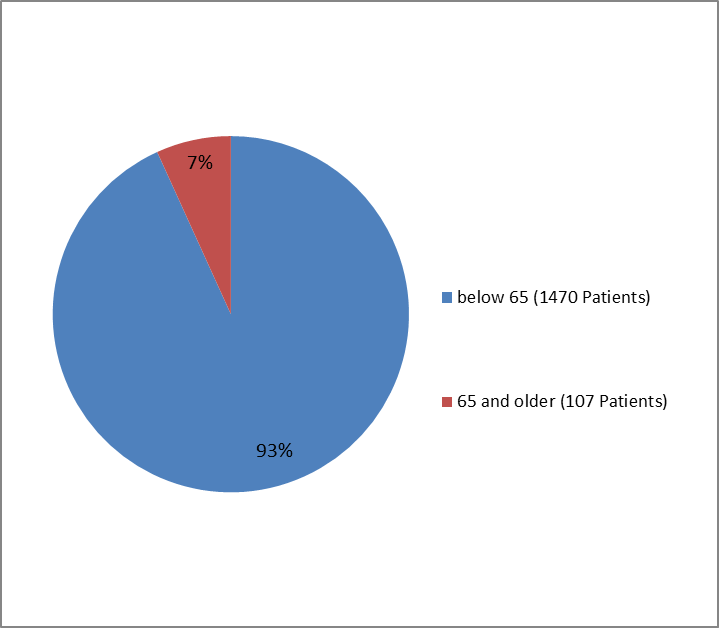
Figure 5. Trials of Patients with Type 1 DM by Age Group
Clinical Trial Data
Figure 6. Trials of Patients with Type 2 DM by Age Group
Clinical Trial Data
Who participated in the trials?
The table below summarizes who participated in 3 pooled clinical trials of patients with type 1 DM.
Table 13. Baseline Demographics for Pooled Clinical Trials in Patients with Type 1 DM
Demographic Parameter |
TRESIBA N=1103 n (%) |
Control N=474 n (%) |
Total N=1577 n (%) |
|---|---|---|---|
| Sex | |||
| Men | 624 (56.6) | 264 (55.7) | 888 (56.3) |
| Women | 479 (43.4) | 210 (44.3) | 689 (43.7) |
| Age Group | |||
| Below 65 years | 1026 (93.0) | 444 (93.7) | 1470 (93.2) |
| 65 years and above | 77 (7.0) | 30 (6.3) | 107 (6.8) |
| Race | |||
| White | 889 (80.6) | 380 (80.2) | 1269 (80.5) |
| Black or African American | 19 (1.7) | 4 (0.8) | 23 (1.5) |
| Asian | 172 (15.6) | 86 (18.1) | 258 (16.4) |
| Other | 23 (2.1) | 4 (0.8) | 27 (1.7) |
| Ethnicity | |||
| Hispanic or Latino | 46 (4.2) | 23 (4.9) | 69 (4.4) |
| Not Hispanic or Latino | 1057 (95.8) | 451 (95.1) | 1508 (95.6) |
Clinical Trial Data
The table below summarizes who participated in 5 pooled clinical trials of patients with Type 2 DM.
Table 14. Baseline Demographics for Pooled Clinical Trials in Patients with Type 2 DM
| Demographic Parameter | TRESIBA N=2716 n (%) |
Control N=1332 n (%) |
Total N=4048 n (%) |
|---|---|---|---|
| Sex | |||
| Men | 1553 (57.2) | 731 (54.9) | 2284 (56.4) |
| Women | 1163 (42.8) | 601 (45.1) | 1764 (43.6) |
| Age Group | |||
| Below 65 years | 2046 (75.3) | 1033 (77.6) | 3079 (76.1) |
| 65 years and above | 670 (24.7) | 299 (22.4) | 969 (23.9) |
| Race | |||
| White | 1918 (70.6) | 905 (67.9) | 2823 (69.7) |
| Black or African American | 183 (6.7) | 98 (7.4) | 281 (6.9) |
| Asian | 558 (20.5) | 296 (22.2) | 854 (21.1) |
| Other | 57 (2.1) | 33 (2.5) | 90 (2.2) |
| Ethnicity | |||
| Hispanic or Latino | 329 (12.1) | 170 (12.8) | 499 (12.3) |
| Not Hispanic or Latino | 2349 (86.5) | 1139 (85.5) | 3488 (86.2) |
| Not applicable | 38 (1.4) | 23 (1.7) | 61 (1.5) |
Not applicable=as defined by the company
Clinical Trial Data
How were the trials designed?
The benefits and side effects of TRESIBA for the treatment of adult patients with type 1 DM were evaluated in two 26-week and one 52-week clinical trials. In these trials, patients were randomly assigned to receive either TRESIBA or another long-acting insulin. TRESIBA was given once-daily either at the same time each day or at any time each day and used along with mealtime insulin.
The benefits and side effects of TRESIBA used along with mealtime insulin or used as another therapy in addition to oral medicines for the treatment of type 2 DM was evaluated in four 26-week and two 52-week clinical trials.
In each trial, the change in HbA1c from the start to finish of the trial was measured.
How were the trials designed?
(no additional information)
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.