Drug Trials Snapshot: PARSABIV
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the PARSABIV Prescribing Information for complete information.
PARSABIV (etelcalcetide)
(PAR-sa-biv)
Amgen Inc.
Approval date: February 7, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
PARSABIV is used to treat high levels of parathyroid hormone (PTH) in adult patients with chronic kidney disease (CKD) on hemodialysis.
How is this drug used?
PARSABIV is given by an injection into the vein three times a week by a health care professional after a hemodialysis session is completed.
What are the benefits of this drug?
PARSABIV lowers the level of parathyroid hormone.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes the efficacy results. This was based on the proportion of patients who had at least 30% decrease in PTH during the efficacy assessment period (EAP) - weeks 20 through 27. The other outcome measures were the proportion of patients with a mean PTH ≤ 300 pg/mL during the EAP, percent change from baseline during the EAP for PTH, corrected serum calcium and serum phosphate levels.
The efficacy analysis was based on Full Analysis Set (FAS) defined as all randomized patients.
Table 2. Effects of PARSABIV on Parathyroid Hormone, Corrected Serum Calcium, and Phosphate Measured at Weeks 20 to 27 in Two 6-Month Placebo-Controlled Trials in Chronic Kidney Disease Patients with Secondary Hyperparathyroidism on Hemodialysis
|
|
Trial 1 |
Trial 2 |
||
|---|---|---|---|---|
|
|
|
|
|
|
|
Parathyroid Hormone |
||||
|
Baseline (pg/mL): |
|
|
|
|
|
Median |
706 |
706 |
740 |
726 |
|
Mean (SE) |
849 (33) |
820 (24) |
845 (29) |
852 (34) |
|
Mean EAP (pg/mL) (SE)f |
424 (37) |
898 (34) |
416 (32) |
971 (46) |
|
Mean Percent Change, % (SE)b,f |
-49.4 (3.4)c |
14.9 (3.6) |
-47.8 (3.7)c |
18.6 (3.5) |
|
Patients with > 30% Reduction in PTH during the EAP, multiple imputation, n (%)a,f |
196 (77)c |
28 (11) |
201 (79)c |
29 (11) |
|
Patients with ≤ 300 pg/mL in PTH during the EAP, n (%)b,f |
131 (52)c |
16 (6) |
142 (56)c |
14 (5) |
|
Corrected Serum Calcium |
||||
|
Mean Baseline (mg/dL) (SE) |
9.7 (0.04) |
9.6 (0.04) |
9.6 (0.04) |
9.7 (0.04) |
|
Mean EAP (mg/dL) (SE)f |
9.0 (0.1) |
9.7 (0.04) |
9.0 (0.1) |
9.7 (0.05) |
|
Mean Percent Change, % (SE)b,f |
-7.0 (0.6)c |
0.9 (0.6) |
-7.0 (0.7)c |
-0.8 (0.6) |
|
Serum Phosphate |
||||
|
Mean Baseline (mg/dL) (SE) |
6.0 (0.1) |
5.8 (0.1) |
5.8 (0.1) |
5.8 (0.1) |
|
Mean EAP (mg/dL) (SE)f |
5.4 (0.1) |
5.5 (0.1) |
5.2 (0.1) |
5.6 (0.1) |
|
Mean Percent Change, % (SE)b,f |
-8.8 (2.5)e |
-3.6 (2.8) |
-7.2 (2.5)d |
-0.3 (2.2) |
|
a Main outcome measure for Trials 1 and 2. |
||||
PARSABIV Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: PARSABIV worked similarly in men and women.
- Race: The majority of patients in the trials were white. Differences in response to PARSABIV among races could not be determined.
- Age: PARSABIV worked similarly in patients above and below age 65.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
Exploratory analyses of subgroup efficacy in pooled Trials 1 and 2 (randomized population) are presented in the figure below.
Figure 4. Treatment Difference in Primary Endpoint by Subgroup
OR=odds ratio
CL=confidence limit
LCL=lower confidence limit
UCL=upper confidence limit
Clinical trial data
What are the possible side effects?
PARSABIV may cause serious side effects including low calcium level in the blood, worsening of heart failure, stomach bleeding and bone disorder. Low blood calcium may cause serious heart rhythm problems, and seizures.
The most common side effects of PARSABIV are decreased blood calcium, muscle spasms, diarrhea, nausea, and vomiting.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in pooled trials based on the safety population defined as all patients who received at least one dose of investigational product.
Table 3 . Adverse Reactions with Frequency ≥ 5% of Patients on Hemodialysis in the PARSABIV Group in Combined Placebo-Controlled Trials
|
Adverse Reaction* |
Placebo (N = 513) |
PARSABIV (N = 503) |
|---|---|---|
|
Blood calcium decreaseda |
10 |
64 |
|
Muscle spasms |
7 |
12 |
|
Diarrhea |
9 |
11 |
|
Nausea |
6 |
11 |
|
Vomiting |
5 |
9 |
|
Headache |
6 |
8 |
|
Hypocalcemiab |
0.2 |
7 |
|
Paresthesiac |
1 |
6 |
|
*Included adverse reactions reported with at least 1% greater incidence in the PARSABIV group compared to the placebo group. |
||
PARSABIV Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients in the trials were white. Differences in side effects among races could not be determined.
- Age: The occurrence of side effects was similar between patients above and below age 65.
Were there any differences in side effects of the clinical trials among sex, race and age groups?
The table below summarizes Hypocalcemia adverse reaction by subgroups. Presented are exploratory results for safety population in combined clinical trials.
Table 4. Hypocalcemia* Adverse Reaction by Subgroups (safety population)
|
|
Trials 1 and 2 combined |
|
|---|---|---|
|
Placebo |
PARSABIV |
|
|
Sex |
||
|
Men |
32/304 (11) |
210/308 (68) |
|
Women |
21/209 (10) |
120/195 (62) |
|
Age (years) |
||
|
<>
|
39/336 (12) |
229/326 (70) |
|
≥65 |
17/177 (8) |
101/177(57) |
|
Race |
||
|
White /Other |
38/364 (11) |
258/368 (70) |
|
Black or African American |
15/149 (10) |
72/135 (53) |
* hypocalcemia =includes PT (preferred term)“blood calcium decreased” and PT “hypocalcemia”
**x/n= number of patents with adverse reaction (x) in the subgroup (n)
Company trial data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved PARSABIV based on evidence from two clinical trials of patients with elevated PTH who were receiving hemodialysis because of chronic kidney failure. The trials were conducted in the United States, Canada, Europe, Israel, Russia, and Australia.
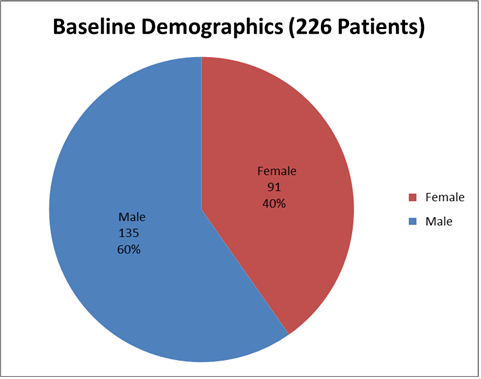
Figure 1 summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
Clinical trial data
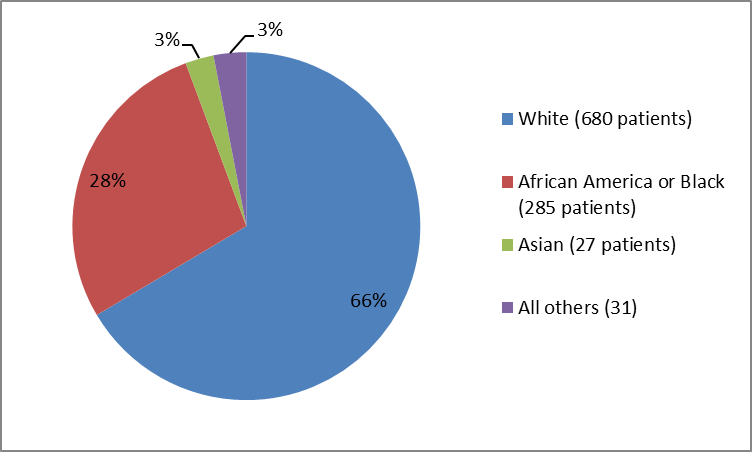
Figure 2 and Table 1 summarize the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
Clinical trial data
Table 1. Baseline Demographics by Race
|
Race |
Number of Patients |
Percentage |
|---|---|---|
|
White |
680 |
66% |
|
Black or African American |
285 |
28% |
|
Asian |
27 |
3% |
|
Native Hawaiian or Other Pacific Islander |
12 |
2% |
|
Other |
16 |
2% |
|
Missing |
3 |
less then 1% |
Clinical trial data
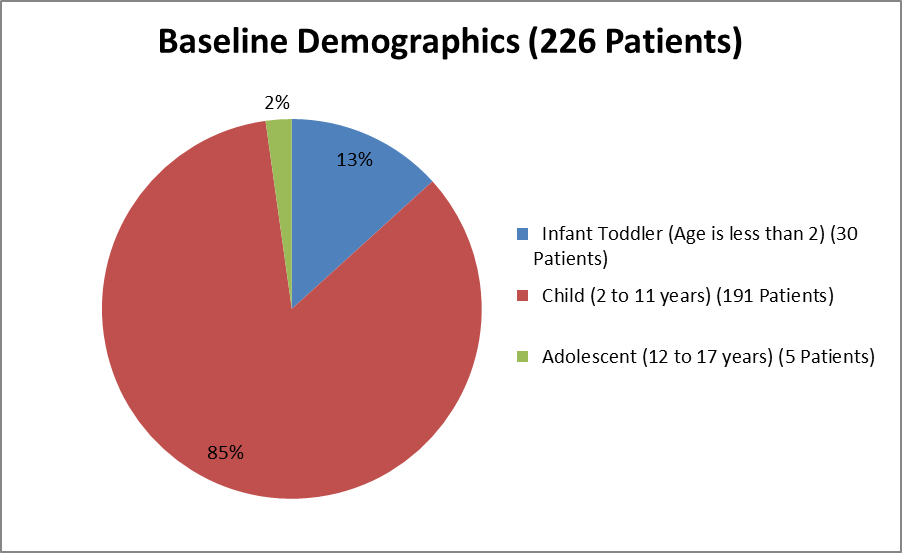
Figure 3 summarizes the percentage of patients by age gropus in the clinical trials.
Figure 3. Baseline Demographics by Age
Clinical trial data
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trials based on randomized population.
Table 5. Baseline Demographics in the Clinical Trials (randomized population)
|
Demographic Parameters |
Trials 1 and 2 (N=1023) |
|---|---|
|
Sex |
|
|
Men |
618 (60) |
|
Women |
405 (40) |
|
Age |
|
|
Median (years) |
58 |
|
Min, Max (years) |
21,93 |
|
Age Group |
|
|
<65> |
666 (65) |
|
≥65 years |
357 (35) |
|
≥75 and above |
138 (13) |
|
Race |
|
|
White |
680 (66) |
|
African American or Black |
285 (28) |
|
Asian |
27 (3) |
|
Native Hawaiian or Other |
12 (1) |
|
Other |
16 (2) |
|
Missing |
3 (<> |
|
Ethnicity |
|
|
Hispanic/Latino |
131 (13) |
|
Not Hispanic/Latino |
889 (87) |
|
Missing |
3 (<> |
|
Region |
|
|
United States |
533 (52) |
|
Europe |
361 (35) |
|
Other |
129 (13) |
Clinical trial data
How were the trials designed?
There were two, 6 months long trials that evaluated the benefits and side effects of PARSABIV.
In both trials, patients were randomly assigned to receive PARSABIV or placebo injections 3 time per week at the end of hemodialysis. Neither the patients nor the health care providers knew which treatment was being given until after the trials were completed.
The benefit of PARSABIV was determined by proportion of patients who had at least 30% decrease in PTH during weeks 20 through 27.
How were the trials designed?
PARABIV trials include two 6-months, double-blind, randomized, placebo-controlled trials. Patients with secondary hyperparathyroidism were administered PARSABIV or placebo at a starting dose of 5 mg three times per week at the end of hemodialysis and titrated every 4 weeks through week 17 to a maximum dose of 15 mg three times per week to achieve target PTH level ≤ 300 pg/mL.
The primary endpoint for both trials was the proportion of patients who achieved a > 30% reduction from baseline in mean PTH during the efficacy assessment period (EAP). The other outcome measures were the proportion of patients with a mean PTH ≤ 300 pg/mL during the EAP, percent change from baseline during the EAP for PTH, serum corrected calcium, and serum phosphate.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.