Drug Trials Snapshot: ODOMZO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the ODOMZO Prescribing Information for complete information.
ODOMZO (sonidegib)
(o-DOM-zo)
Novartis Pharmaceuticals Corporation
Approval date: July 24, 2015
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ODOMZO is a drug for the treatment of basal cell carcinoma. It is approved to treat patients whose basal cell carcinoma is locally advanced (abbreviated laBCC) and whose cancer has returned after surgery, or who cannot have radiation or surgery to remove all the cancer.
How is this drug used?
ODOMZO is a capsule that is taken once a day.
What are the benefits of this drug?
ODOMZO led to tumor shrinkage or tumor disappearance.
What are the benefits of this drug (results of trials used to assess efficacy)?
Out of 79 patients who were treated with ODOMZO 200 mg daily, 66 had laBCC and 13 had metastatic basal cell carcinoma (mBCC). The confirmed objective response rate (ORR) for the 66 patients with laBCC receiving ODOMZO was 58% (95% CI: 45, 70) at a timepoint when all patients had been followed on the study for at least 12 months if they had not discontinued ODOMZO earlier.
ORR was determined by blinded central review according to a protocol-specific modified Response Evaluation Criteria in Solid Tumors (mRECIST) which involved an integrated assessment of radiology, photography, and tumor biopsy results.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The number of patients in the trial was small. ODOMZO appeared to work similarly in men and women.
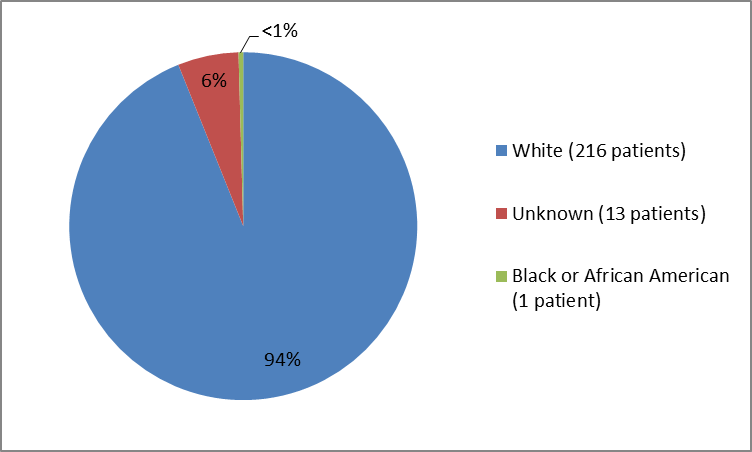
- Race: Most patients in the trials were white. Differences in response to ODOMZO among races could not be determined.
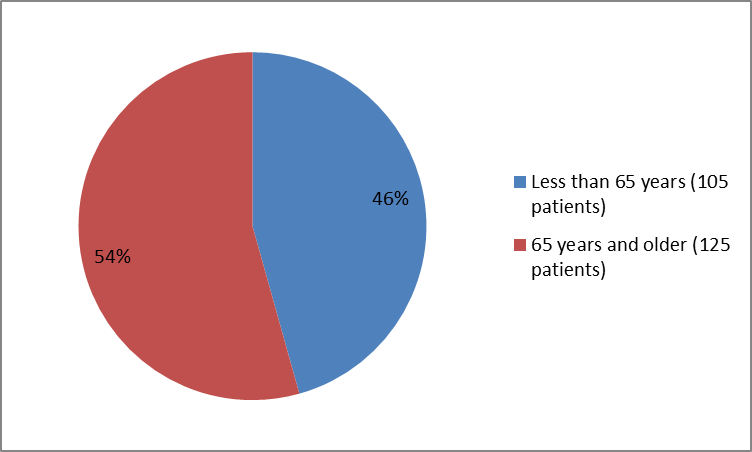
- Age: The number of patients in the trial was small. ODOMZO appeared to work similarly in patients younger than 65 and patients 65 and older.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes the primary endpoint results by subgroup.
Table 2. Subgroup Analysis of Primary Endpoint
| Demographic Parameters | Treatment Groups | |
|---|---|---|
| ODOMZO 200 mg daily N=79 n (%) |
ODOMZO 800 mg daily1 N=151 n (%) |
|
| Disease Stage | ||
| Locally advanced BCC | 38/66 (58) | 56/128 (44) |
| Metastatic BCC | 1/13 (8) | 4/23 (17) |
| Sex | ||
| Female | 18/31 (58) | 25/55 (46) |
| Male | 21/48 (44) | 35/96 (37) |
| Age Group | ||
| <65 | 19/32 (59) | 32/73 (44) |
| >=65 years | 20/47 (43) | 28/78 (36) |
| Race | ||
| White | 36/71 (51) | 58/145 (40) |
| Non-White* | 3/8 (38) | 2/6 (33) |
1not an approved dose
*African-American and Unknown race combined
Source: Company Trial Data
What are the possible side effects?
The most common side effects were:
- muscle cramps
- hair loss
- disturbed sense of taste
- tiredness
- nausea and vomiting
- muscle pain
- diarrhea
- weight loss
- decreased appetite
- abdominal pain and diarrhea
- headache
- pain
- itching
When used by a pregnant woman, ODOMZO can cause a baby to die before it is born (be stillborn) or lead to severe birth defects. Women should avoid pregnancy during treatment and for 20 months after treatment is finished. Men who take ODOMZO should use a condom during treatment and for 8 months after treatment is finished. Also during this time, sperm should not be donated.
ODOMZO can cause serious injury to the muscles that can lead to kidney damage.
What are the possible side effects?
The table below summarizes side effects in the clinical trial. The population represented is any patient who received at least one dose of ODOMZO.
Table 3. Adverse Drug Reactions in ≥10% of ODOMZO‑Treated Patients
| Adverse Reactions | ODOMZO (N=79) |
|
|---|---|---|
| All Grades a % | Grade 3 % | |
| Musculoskeletal and connective tissue disorders | ||
| Muscle spasms | 54 | 3 |
| Musculoskeletal pain | 32 | 1 |
| Myalgia | 19 | 0 |
| Skin and subcutaneous tissue disorder | ||
| Alopecia | 53 | 0 |
| Pruritus | 10 | 0 |
| Nervous system disorders | ||
| Dysgeusia | 46 | 0 |
| Headache | 15 | 1 |
| General disorders and administration site conditions | ||
| Fatigue | 41 | 4 |
| Pain | 14 | 1 |
| Gastrointestinal disorders | ||
| Nausea | 39 | 1 |
| Diarrhea | 32 | 1 |
| Abdominal pain | 18 | 0 |
| Vomiting | 11 | 1 |
| Investigations | ||
| Decreased weight | 30 | 3 |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 23 | 1 |
Source: ODOMZO Prescribing Information Section 6, Table 1
a No Grade 4 adverse reactions were reported.
Were there any differences in side effects among sex, race and age?
- Sex: The number of patients in the trial was small. Differences in side effects between men and women could not be determined.
- Race: Most of the patients in the trials were White. Differences in side effects among races could not be determined.
- Age: The number of patients in the trial was small. However, certain side effects—called serious adverse events*—were seen more frequently in patients 65 years and older.
*defined as an adverse event that is fatal or life-threatening, results in persistent or significant disability, constitutes a congenital anomaly, requires inpatient hospitalization or prolongs an existing hospitalization, or is considered medically significant because it requires medical or surgical intervention to prevent an undesirable outcome.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
According to the ODOMZO Prescribing Information, Section 8.5, certain side effects—called serious adverse events*—were seen more frequently in patients 65 years and above.
* defined as an adverse event that is fatal or life-threatening, results in persistent or significant disability, constitutes a congenital anomaly, requires inpatient hospitalization or prolongs an existing hospitalization, or is considered medically significant because it requires medical or surgical intervention to prevent an undesirable outcome.
WHO WAS IN THE CLINICAL TRIALS
Who participated in the clinical trials?
The FDA approved ODOMZO based on evidence from a clinical trial that enrolled 230 patients with either locally advanced basal cell carcinoma (194 patients) or with wide-spread basal cell carcinoma (36 patients). The trial was conducted in Australia, Europe, and North America.
The figure below summarizes how many men and women were enrolled (230 patients) in the clinical trial.
Figure 1. Baseline Demographics by Sex
Source: Company Trial Data
The figure and table below summarize the percentage of patients by race enrolled in the clinical trial.
Figure 2. Baseline Demographics by Race
<1%=less than="" 1%="">
Source: Company Trial Data
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 216 | 94% |
| Unknown | 13 | 6% |
| Black or African American | 1 | less than 1% |
Source: Company Trial Data
The figure below summarizes the percentage of patients by age enrolled in the clinical trial.
Figure 3. Baseline Demographics by Age
Source: Company Trial Data
Who participated in the trials?
The table below summarizes baseline demographics for the enrolled trial population.
Table 4. Baseline Demographics for the Trial
| Demographic Parameter | Treatment Groups | Total N=230 n (%) |
|
|---|---|---|---|
| ODOMZO 1 N=79 n (%) |
ODOMZO 800 mg daily 2 N=151 n (%) |
||
| Sex | |||
| Male | 48 (61) | 96 (64) | 144 (63) |
| Female | 31 (39) | 55 (36) | 86 (37) |
| Age | |||
| Mean years (SD) | 65.6 (15.7) | 63.6 (14.6) | 64.3 (15) |
| Median (years) | 67 | 65 | 66 |
| Min, Max (years) | 25.0, 92.0 | 24.0, 93.0 | 24.0, 93.0 |
| Age Group | |||
| <65> | 32 (41) | 73 (48) | 105 (46) |
| >=65 years | 47 (59) | 78 (52) | 125 (54) |
| Race | |||
| White | 71 (90) | 145 (96) | 216 (94) |
| Black or African American | 0 (0) | 1 (1) | 1 (<> |
| Unknown | 8 (10) | 5 (3) | 13 (6) |
| Ethnicity | |||
| Hispanic or Latino | 3 (4) | 1 (1) | 4 (2) |
| Not Hispanic or Latino | 65 (82) | 131 (87) | 196 (85) |
| Unknown | 11 (14) | 19 (12) | 30 (13) |
| Region | |||
| Australia | 5 (6) | 7 (5) | 12 (5) |
| Europe | 45 (57) | 83 (55) | 128 (56) |
| North America | 29 (37) | 61 (40) | 90 (39) |
1includes 13 patients with metastatic BCC
2not approved dose
Source: Company Trial Data
How were the trials designed?
There was one trial that evaluated the benefits and side effects of ODOMZO at one of two doses. In the trial, patients were randomly assigned to receive either ODOMZO 200 mg once a day or a higher dose of ODOMZO once a day. All patients were followed for at least 12 months. Neither the patients nor the health care providers knew which dose of ODOMZO was being given.
The trial measured the proportion of patients who experienced partial shrinkage or complete disappearance of their tumors while receiving ODOMZO.
How were the trials designed?
A single, multicenter, double-blind, randomized, two-arm, non-comparative trial involving 230 patients with either laBCC (n=194) or metastatic BCC (n=36) was conducted. Patients were randomly assigned to receive either ODOMZO 200 mg or 800 mg daily. Patients were followed for at least 12 months.
The primary efficacy outcome measure of the trial was objective response rate (ORR). ORR was determined by blinded central review according to a protocol-specific modified Response Evaluation Criteria in Solid Tumors (mRECIST), using an integrated composite response based on radiographic (MRI), photographic (digital clinical photography), and histological data.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION