Drug Trials Snapshot: LYBALVI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the LYBALVI Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
LYBALVI (olanzapine and samidorphan)
(lee bawl’ vee)
Alkermes Inc
Original Approval date: May 28, 2021
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
LYBALVI is a combination of olanzapine, an atypical antipsychotic, and samidorphan, an opioid antagonist. LYBALVI is used for:
- Schizophrenia in adults
- Alone for short-term (acute) or maintenance treatment of manic or mixed episodes that happen with bipolar I disorder
- In combination with valproate or lithium to treat manic or mixed episodes that happen with bipolar I disorder
Schizophrenia is a brain disorder with symptoms that include hearing voices, believing that other people are reading one’s mind or controlling their thoughts, and being suspicious or withdrawn.
Bipolar disorder, also known as manic-depressive illness, is a brain disorder that causes unusual shifts in mood, energy, activity levels and the ability to carry out day-to-day tasks.
How is this drug used?
LYBALVI is a tablet taken by mouth once a day with or without food. Do not divide tablets or combine strengths.
Who participated in the clinical trials?
The benefit of LYBALVI was assessed with evidence from two clinical trials. Trial 1 included 397 patients (Full Analysis Set) with schizophrenia and was conducted at 38 sites in the United States, Bulgaria, Serbia, and Ukraine. Trial 2 included 538 patients (Full Analysis Set) with schizophrenia and was conducted at 64 sites in the United States.
The safety of LYBALVI was supported by a pooled analysis that included patients from Trials 1 and 2, two long-term follow up safety studies, and one phase 2 study.
What are the benefits of this drug?
The olanzapine component of LYBALVI improves symptoms of schizophrenia and bipolar I disorder. In a 24-week trial, patients with schizophrenia who took LYBALVI gained weight but to a lesser degree than those who took olanzapine alone. The effect of samidorphan on weight gain is expected to be similar in patients with bipolar I disorder.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 1 summarizes analysis results of the primary efficacy endpoint for Trial 1. The primary efficacy endpoint was the change from baseline in Positive and Negative Syndrome Scale (PANSS) at Week 4. The analysis population is the full analysis set (FAS) and consists of all randomized patients who received at least one dose of study drug and had at least one post-randomization efficacy evaluation for PANSS total score.
Table 1. Summary of Efficacy Results - Schizophrenia Symptoms (FAS Population, Trial 1)
|
Treatment Group |
Total PANSS Score |
||
|---|---|---|---|
|
Baseline Mean Score (SD) |
LS Mean Change From Baseline (SE) |
Placebo-Subtracted Differencea (95% CI) |
|
|
LYBALVI |
101.8 (11.6) |
-23.9 (1.3) |
-6.4 (-10.0, -2.8) |
|
Placebo (N=133) |
102.7 (11.9) |
-17.5 (1.3) |
- |
Source: LYBALVI Prescribing Information
a Difference (drug minus placebo) in least squares mean change from baseline. A negative value for the placebo subtracted difference represents improvement.
Abbreviations: CI, confidence interval; FAS, full analysis set; LS, least squares; PANSS, Positive and Negative Syndrome Scale; SD, standard deviation; SE, standard error
Table 2 summarizes analysis results of the co-primary efficacy endpoints for Trial 2. The co-primary endpoints were the percent change from baseline in body weight and the proportion of subjects with 10% or more weight gain from baseline, both at Week 24. The analysis population is the FAS and consists of all randomized patients who received at least one dose of study drug and had at least one post-baseline weight assessment.
Table 2. Summary of Efficacy Results – Weight Mitigation (FAS Population, Trial 2)
|
Treatment Group |
% Change From Baseline in Body Weight |
≥10% Body Weight Gain |
|||
|---|---|---|---|---|---|
|
Baseline Mean, kg (SD) |
LS Mean |
Olanzapine-Subtracted Difference (95% CI) |
Percentage of Patients |
Olanzapine- Subtracted Risk Difference (95% CI) |
|
|
LYBALVI |
77.0 |
4.2 |
-2.4 |
17.8 |
-13.7 |
|
Olanzapine |
77.5 |
6.6 |
- |
29.8 |
- |
Source: LYBALVI Prescribing Information
Abbreviations: CI: confidence interval; FAS, full analysis set; LS: least squares; SD: standard deviation; SE: standard error
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Schizophrenia Symptoms
- Sex: LYBALVI worked similarly in males and females.
- Race: Subgroup analyses by race were inconclusive due to other factors that are likely confounded with the analyses. Differences in how well LYBALVI worked in races studied could not be determined.
- Age: Few patients older than 65 years old were enrolled in the study. Differences in how well LYBALVI worked in patients younger and older than 65 years of age could not be determined.
Weight Mitigation
- Sex: LYBALVI worked similarly in males and females.
- Race: LYBALVI worked similarly in all races studied.
- Age: Patients older than 55 years of age did not enroll in the study. Differences in how well LYBALVI worked in patients younger and older than 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3 and Table 4 below summarize the responses to LYBALVI by sex and race subgroups for the FAS population which includes all randomized patients who received at least one dose of study drug and had at least one post-randomization measurement for PANSS total score (Trial 1) or weight (Trial 2). Of note, the comparisons of sex and race were not powered to determine differences between groups for changes in PANSS total score or weight.
Table 3. Subgroup Analysis of Change From Baseline in PANSS Total Score at Week 4 by Sex and Race (Schizophrenia Symptoms, Trial 1)
|
Subgroup |
N |
Placebo-Subtracted Difference in LS Mean Change From Baseline (95% CI) |
|
|---|---|---|---|
|
LYBALVI |
Placebo |
||
|
Sex |
|||
|
Male |
83 |
78 |
-6.7 (-11.3, -2.2) |
|
Female |
49 |
55 |
-6.2 (-12.2, -0.3) |
|
Race |
|||
|
Black/AA |
41 |
38 |
3.0 (-4.1, 10.1) |
|
White |
86 |
90 |
-9.5 (-13.6, -5.4) |
Source: FDA Multi-Disciplinary Review and Evaluation
Abbreviations: CI, confidence interval; LS, least-squares; N, sample size; PANSS, Positive and Negative Syndrome Scale
Table 4. Subgroup Analysis of Percent Change From Baseline in Body Weight at Week 24 by Sex and Race (Weight Mitigation, Trial 2)
|
Subgroup |
N |
Olanzapine-Subtracted Difference in LS Mean % Change From Baseline (95% CI) |
|
|---|---|---|---|
|
LYBALVI |
Olanzapine |
||
|
Sex |
|||
|
Male |
188 |
203 |
-2.73 (-4.45, -1.01) |
|
Female |
75 |
69 |
-1.53 (-4.43, -1.38) |
|
Race |
|||
|
Black/AA |
196 |
196 |
-2.37 (-4.10, -0.63) |
|
Other |
70 |
76 |
-2.41 (-5.28, 0.46) |
Source: FDA Multi-Disciplinary Review and Evaluation
Abbreviations: AA, African American; CI, confidence interval; LS, least squares; N, sample size
What are the possible side effects?
LYBALVI can cause serious side effects including increased risk of stroke (cerebrovascular problems) in elderly people with dementia-related psychosis that can lead to death, opioid withdrawal and risk of life-threatening opioid overdose, neuroleptic malignant syndrome, drug reaction with eosinophilia and systemic symptoms (DRESS), problems with your metabolism, uncontrolled body movements (tardive dyskinesia), decreased blood pressure (orthostatic hypotension) and fainting, falls, low white blood cell count, difficulty swallowing, seizures (convulsions), problems controlling your body temperature so that you feel too warm, increased prolactin levels in your blood, changes in thinking and movement, and worsening of anticholinergic medication effects or symptoms of being unable to empty your bladder, enlarged prostate, constipation, intestinal blockage, or related conditions.
The most common side effects of LYBALVI:
- When used to treat people with schizophrenia include weight gain, sleepiness, dry mouth, and headache.
- When used alone to treat people with manic or mixed episodes that happen with bipolar I disorder include weakness, dry mouth, constipation, increased appetite, sleepiness, dizziness, and shaking.
- When used in combination with lithium or valproate to treat people with mixed or manic episodes that happen with bipolar I disorder include dry mouth, abdominal discomfort, weight gain, increased appetite, dizziness, back pain, constipation, problems speaking, mouth-watering, memory problems, numbness and tingling in your arm and legs.
What are the possible side effects (results of trials used to assess safety)?
Table 5 below summarizes adverse reactions in the Trial 1 Safety population defined as all patients who received at least one dose of study drug.
Table 5. Adverse Reactions Reported in ≥2% of LYBALVI-Treated Patients and Greater Than Placebo in a 4-Week Schizophrenia Trial (Safety Population): Trial 1
|
Adverse Reaction |
Placebo |
LYBALVI |
|---|---|---|
|
Weight increased |
3 |
19 |
|
Somnolence |
2 |
9 |
|
Dry mouth |
1 |
7 |
|
Headache |
3 |
6 |
|
Blood insulin increased |
1 |
3 |
|
Sedation |
0 |
2 |
|
Dizziness |
1 |
2 |
|
Neutrophil count decreased |
0 |
2 |
Source: LYBALVI Prescribing Information
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The occurrence of side effects was similar between White and Black patients. There were too few patients of other races to make comparisons.
- Age: The majority of patients were adults between 18 and 64 years of age. There were not enough patients 65 or older to determine whether there was any difference in side effects between older and younger patients.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
To determine if there were any differences in the safety of LYBALVI between subgroups, a pooled analysis was used. This analysis pooled data across studies that supported the safety of LYBALVI, including Trials 1 and 2 above, the long-term follow-up safety studies, and one Phase 2 study.
Table 6. Any Treatment-Emergent Adverse Events by Subgroup (Safety Population)
|
Subgroup |
Treatment-Emergent Adverse Event |
|---|---|
|
Sex |
|
|
Male (N=569) |
393 (69.1) |
|
Female (N=262) |
170 (64.9) |
|
Race |
|
|
Black (N=428) |
319 (74.5) |
|
White (N=369) |
219 (59.3) |
|
Other (N=34) |
25 (73.5) |
Source: Adapted from FDA Review
DEMOGRAPHICS SNAPSHOT
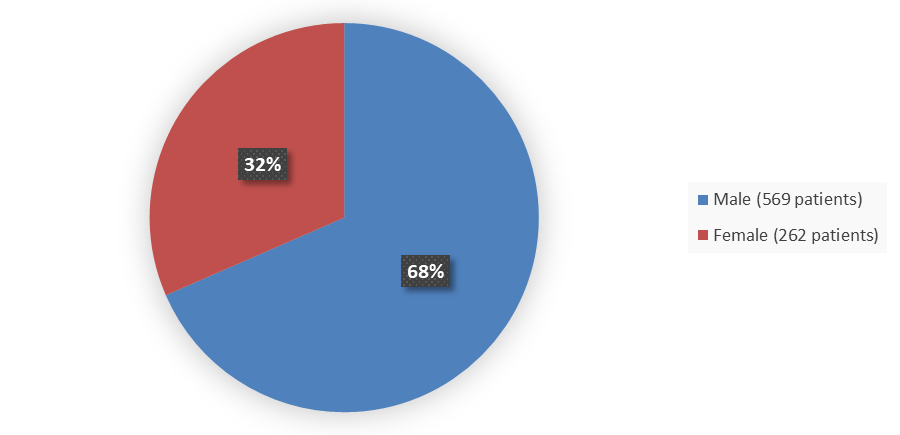
Figure 1 summarizes how many patients by sex were enrolled in the combined clinical trials used to evaluate the efficacy of LYBALVI.
Figure 1. Baseline Demographics by Sex (Safety Population)
Source: Adapted from FDA Review
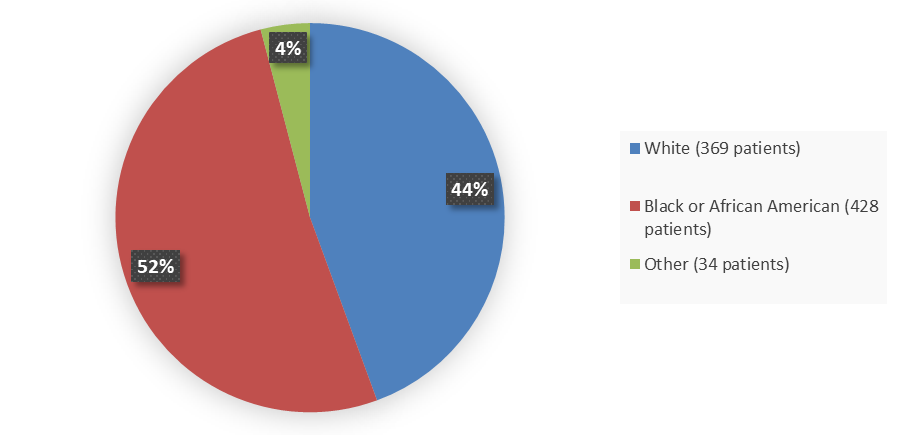
Figure 2 summarizes the percentage of patients by race enrolled in the combined clinical trials used to evaluate the efficacy of LYBALVI.
Figure 2. Baseline Demographics by Race (Safety Population)
Source: Adapted from FDA Review
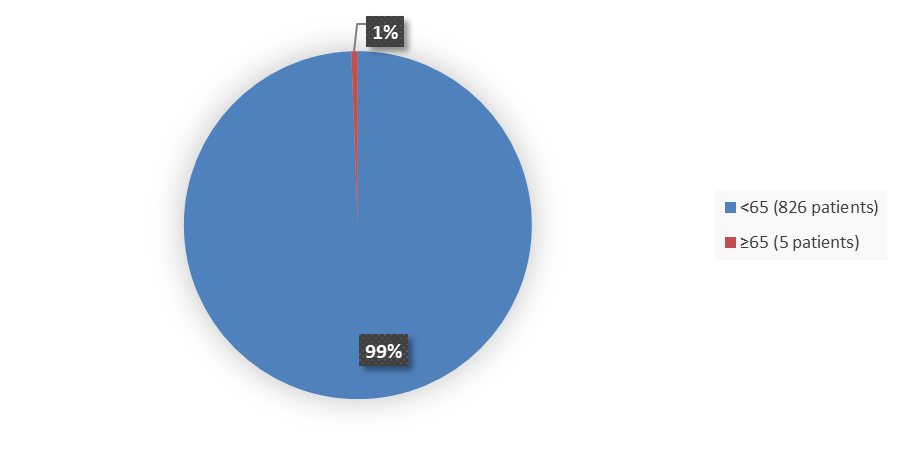
Figure 3 summarizes how many patients by age were in the combined trials used to evaluate the safety of LYBALVI.
Figure 3. Baseline Demographics by Age (Safety Population)
Source: Adapted from FDA Review
Who participated in the trials?
Table 7. Demographics and Other Baseline Characteristics (Safety Population)
|
Demographic |
LYBALVI |
|---|---|
|
Age, years |
|
|
Mean (SD) |
41.4 (10.78) |
|
Median |
42.0 |
|
Min, max |
|
|
Sex, n (%) |
|
|
Male |
569 (68.5) |
|
Female |
262 (31.5) |
|
Race, n (%) |
|
|
American Indian or Alaska Native |
4 (0.5) |
|
Asian |
10 (1.2) |
|
Black or African American |
428 (51.5) |
|
Native Hawaiian or other Pacific Islander |
4 (0.5) |
|
White |
369 (44.4) |
|
Other |
8 (1.0) |
|
Multiple races |
8 (1.0) |
|
Ethnicity, n (%) |
|
|
Hispanic or Latino |
70 (8.4) |
|
Not Hispanic or Latino |
761 (91.6) |
|
Region |
|
|
United States |
593 (71.4) |
|
Non-United States |
238 (28.6) |
Source: Adapted from FDA review
Abbreviations: SD, standard deviation
How were the trials designed?
LYBALVI was evaluated in two clinical trials of 964 patients with schizophrenia.
The benefit of LYBALVI for the treatment of schizophrenia was evaluated in Trial 1 (NCT02634346). Patients were given daily doses of either LYBALVI, olanzapine, or placebo for four weeks. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed. The antipsychotic efficacy of LYBALVI was assessed based on change from baseline in schizophrenia symptoms in each treatment group (LYBALVI, olanzapine, or placebo).
The benefit of LYBALVI for weight changes was evaluated in Trial 2 (NCT02694328). Patients were given either LYBALVI or olanzapine, daily, for 24 weeks. Neither the patients nor the health care providers knew which treatment was given until after the trial was completed. The efficacy of LYBALVI for the evaluation of weight changes was assessed based on percent change from baseline body weight in each treatment group (LYBALVI or olanzapine).
The benefit of LYBALVI in the treatment of adult patients with bipolar I disorder is based on adequate and well-controlled studies of orally administered olanzapine (a component of LYBALVI).
How were the trials designed?
Trial 1 was a 4-week, randomized, double-blind, placebo- and active-controlled study that enrolled adult patients with an acute exacerbation of schizophrenia. The primary endpoint was the change from baseline to Week 4 in PANSS total score. The PANSS is a 30-item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); the total PANSS scores range from 30 to 210.
Trial 2 was a 24-week randomized, double-blind, active-controlled study that enrolled adult patients with schizophrenia who were suitable for outpatient treatment. In Trial 2 there were co‑primary endpoints focusing on change in body weight. The first was percent change in body weight at Week 24 and the second was the proportion of patients who gained ≥10% of their body weight at Week 24. The proportions of patients who discontinued study drug in the 24-week trial was 36% for both LYBALVI and olanzapine-treated groups resulting in statistical imputation of missing data. Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to LYBALVI is unknown.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.