Drug Trials Snapshot: KISQALI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the KISQALI Package Insert for complete information.
KISQALI (ribociclib)
(kis kah' lee)
Novartis Pharmaceuticals Corporation
Approval date: March 13, 2017
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
KISQALI is a drug for treatment of a specific form of advanced breast cancer called hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer in women who have gone through menopause.
KISQALI is taken with another type of drug (an aromatase inhibitor) used to treat breast cancer.
How is this drug used?
Three KISQALI tablets are taken in combination with letrozole (an aromatase inhibitor drug) once daily for 21 consecutive days followed by 7 days off treatment.
What are the benefits of this drug?
Women taking KISQALI and letrozole experienced a longer time period before their tumors worsened, in comparison to women who took placebo and letrozole.
Information on overall survival of these women is not available at this time.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 shows the time of Progression Free Survival for both arms of Trial 1.
Table 2. Efficacy Results – Trial 1 (Investigator Assessment, Intent-to-Treat Population)
| KISQALI +letrozole | Placebo + letrozole | |
|---|---|---|
| Progression-free survival | N = 334 | N = 334 |
| Events (%) | 93 (27.8) | 150 (44.9) |
| Median (months, 95% CI) | NR (19.3 – NR) | 14.7 (13.0 – 16.5) |
| Hazard Ratio (95% CI) | 0.556 (0.429 to 0.720 ) | |
| p-value | > | |
| Overall Response Rate | N=256 | N=245 |
| Patients with measurable disease (95% CI) | 52.7 (46.6, 58.9) | 37.1 (31.1, 43.2) |
| a p-value estimated from one-sided log-rank test NR = not reached | ||
KISQALI Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The trial included only women therefore sex differences cannot be determined.
- Race: The majority of patients were white. The number of patients in other races was limited; therefore, differences in response among races could not be determined.
- Age: KISQALI worked similarly in patients above and below 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
Table 3 shows the efficacy results by relevant demographic and geographic subgroups.
Table 3. Progression-Free Survival by Subgroup Analysis
| N | HR | 95% CI | |
|---|---|---|---|
| Age | |||
| 65="" years=""> | 373 | 0.52 | 0.38, 0.72 |
| ≥ 65 Years Old | 295 | 0.61 | 0.40, 0.94 |
| Race | |||
| White | 549 | 0.61 | 0.46, 0.82 |
| Black or African American | 17 | 0.32 | 0.02, 3.5 |
| Asian | 51 | 0.44 | 0.19, 1.00 |
| Geographic Region | |||
| United States | 213 | 0.46 | 0.29, 0.74 |
| Elsewhere | 455 | 0.60 | 0.44, 0.82 |
HR=hazard ratio; CI=confidence interval
FDA Review
What are the possible side effects?
KISQALI may cause serious side effects, including heart rhythm problems and liver damage.
The most common side effects include low level of infection-fighting white blood cells (neutropenia), nausea, tiredness, diarrhea, low level of white blood cells (leukopenia), hair loss, vomiting, constipation, headache and back pain.
What are the possible side effects (results of trials used to assess safety)?
Table 4 below summarizes common adverse reactions at all grades of severity.
Table 4. Adverse Reactions Occurring in ≥ 10% and ≥ 2% higher than Placebo Arm in Trial 1 (All Grades)
| KISQALI + letrozole N=334 | Placebo + letrozole N=330 | |||||
|---|---|---|---|---|---|---|
| All Grades | Grade 3 | Grade 4 | All Grades | Grade 3 | Grade 4 | |
| Adverse drug reactions | % | % | % | % | % | % |
| Infections and Infestations | ||||||
| Urinary tract infection | 11 | 1 | 0 | 8 | 0 | 0 |
| Blood and lymphatic system disorders | ||||||
| Neutropenia | 75 | 50 | 10 | 5 | 1 | 0 |
| Leukopenia | 33 | 20 | 1 | 1 | > | 0 |
| Anemia | 18 | 1 | > | 5 | 1 | 0 |
| Lymphopenia | 11 | 6 | 1 | 2 | 1 | 0 |
| Metabolism and nutrition disorders | ||||||
| Decreased appetite | 19 | 2 | 0 | 15 | > | 0 |
| Nervous system disorders | ||||||
| Headache | 22 | > | 0 | 19 | > | 0 |
| Insomnia | 12 | > | 0 | 9 | 0 | 0 |
| Respiratory, thoracic and mediastinal disorders | ||||||
| Dyspnea | 12 | 1 | 0 | 9 | 1 | 0 |
| Musculoskeletal and connective tissue disorders | ||||||
| Back pain | 20 | 2 | 0 | 18 | > | 0 |
| Gastrointestinal disorders | ||||||
| Nausea | 52 | 2 | 0 | 29 | 1 | 0 |
| Diarrhea | 35 | 1 | 0 | 22 | 1 | 0 |
| Vomiting | 29 | 4 | 0 | 16 | 1 | 0 |
| Constipation | 25 | 1 | 0 | 19 | 0 | 0 |
| Stomatitis | 12 | > | 0 | 7 | 0 | 0 |
| Abdominal pain | 11 | 1 | 0 | 8 | 0 | 0 |
| Skin and subcutaneous tissue disorders | ||||||
| Alopecia | 33 | 0 | 0 | 16 | 0 | 0 |
| Rash | 17 | 1 | 0 | 8 | 0 | 0 |
| Pruritus | 14 | 1 | 0 | 6 | 0 | 0 |
| General disorders and administration site conditions | ||||||
| Fatigue | 37 | 2 | > | 30 | 1 | 0 |
| Pyrexia | 13 | > | 0 | 6 | 0 | 0 |
| Edema peripheral | 12 | 0 | 0 | 10 | 0 | 0 |
| Investigations | ||||||
| Abnormal liver function tests1 | 18 | 8 | 2 | 6 | 2 | 0 |
| Grading according to CTCAE 4.03 (Common Terminology Criteria for Adverse Events) 1 abnormal liver function tests: ALT increased, AST increased, blood bilirubin increased | ||||||
KISQALI Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The trial included only women therefore sex differences cannot be determined.
- Race: The majority of patients were white. The number of patients in other races was limited; therefore, differences in side effects among races could not be determined.
- Age: The incidence of overall side effects was similar in patients above and below 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Analysis of side effects by subgroup was limited to age due to the fact that all patients in the trial were women and vast majority were white.
Table 5. Summary of Treatment-Emergent Adverse Events by Age Group
| KISQALI plus letrozole N=334 | Placebo plus letrozole N=330 | |||
|---|---|---|---|---|
| > N=184 | ≥65 N=150 | > N=186 | ≥65 N=144 | |
| Patients with AEs | 181 (98.4%) | 148 (98.7%) | 181 (97.3%) | 139 (96.5%) |
| Grade 3 or 4 AEs | 141 (76.6%) | 130 (86.7%) | 52 (28.0%) | 56 (38.9%) |
| SAE | 33 (17.9%) | 38 (25.3%) | 22 (11.8%) | 17 (11.8%) |
AE=adverse event; SAE=serious adverse event
FDA review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved KISQALI based on evidence from a clinical trial of 668 women with HR-positive, HER2-negative advanced breast cancer who had not received any previous treatment for advanced disease. The trial was conducted in Europe, North America, Asia, Latin America, South America and Australia.
Figure 1 summarizes how many women were in the clinical trial.
Figure 1. Baseline Demographics by Sex
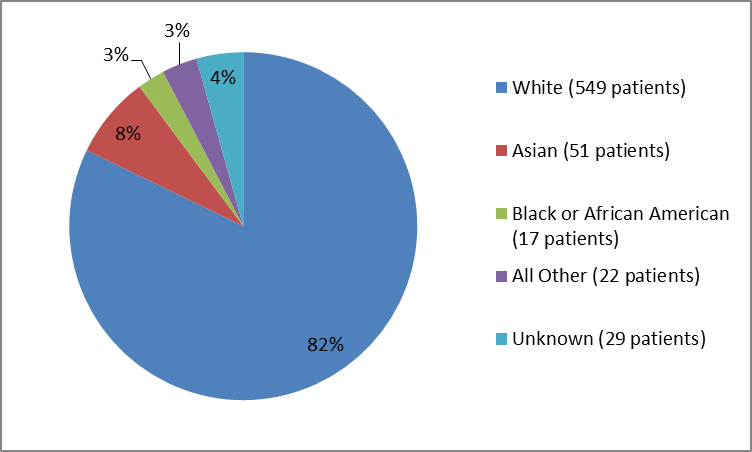
Figure 2 and Table 1 summarize patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 549 | 82 |
| Asian | 51 | 8 |
| Black or African American | 17 | 3 |
| American Indian or Alaska Native | 1 | (less than 1) |
| Native Hawaiian or Other Pacific Islander | 1 | (less than 1) |
| Other | 20 | 3 |
| Unknown | 29 | 4 |
FDA Review
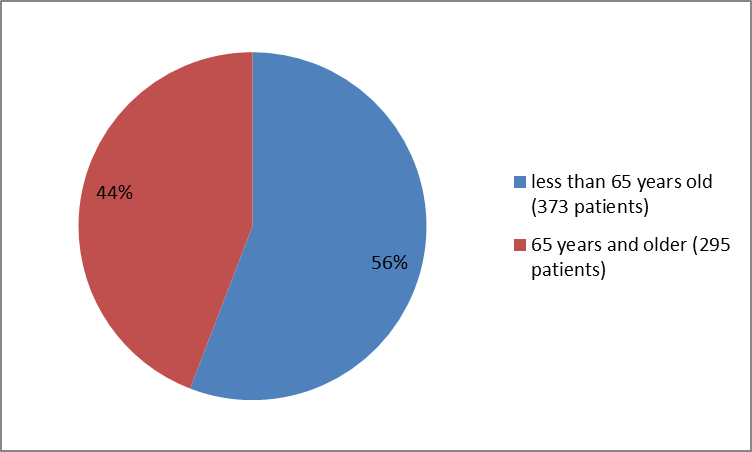
Figure 3 summarizes patients by age in the clinical trial.
Figure 3. Baseline Demographics by Age
Who participated in the trials?
The demographic characteristics of randomized population are summarized in Table 6.
Table 6. Baseline Demographic Characteristics
| KISQALI plus letrozole (N=334) | Placebo plus letrozole (N=334) | Total (N=668) | ||
|---|---|---|---|---|
| Sex, n (%) | ||||
| Women | 334 (100) | 334 (100) | 668 (100) | |
| Age (years) | ||||
| Median (range) | 62 (23 to 91) | 63 (29 to 88) | 62 (23-91) | |
| Age Group (years), n (%) | ||||
| > | 184 (55) | 189 (57) | 373 (56) | |
| > 65 | 150 (45) | 145 (43) | 295 (44) | |
| Race, n (%) | ||||
| White | 269 (81) | 280 (84) | 549 (82) | |
| Black or African American | 10 (3) | 7 (2) | 17 (3) | |
| Asian | 28 (8) | 23 (7) | 51 (8) | |
| American Indian or Alaska Native | 1 (> | 0 | 1(> | |
| Native Hawaiian or Other Pacific Islander | (> | 0 | 1(> | |
| Other | 12(4) | 8 (2) | 20 (3) | |
| Unknown | 13 (4) | 16 (5) | 29 (4) | |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 27 (8) | 37 (11) | 64 (10) | |
| Not Hispanic or Latino | 185 (55) | 167 (50) | 352 (53) | |
| Not reported | 75 (23) | 77 (23) | 152 (23) | |
| Unknown | 47 (14) | 53 (16) | 100 (15) | |
| Region, n (%) | ||||
| North America | 108 (32) | 121 (36) | 229 (34) | |
| Europe | 150 (45) | 146 (44) | 296 (44) | |
| Asia | 35 (11) | 33 (10) | 68 (10) | |
| Latin America | 7 (2) | 7 (2) | 14 (2) | |
| Other | 34 (10) | 27 (8) | 61 (9) | |
Adapted from FDA Review
How were the trials designed?
There was one trial that enrolled postmenopausal women with HR-positive, HER2-negative, advanced breast cancer who received no prior therapy for advanced disease.
The trial compared patients who were randomly assigned to take either a combination of KISQALI and letrozole or placebo and letrozole. Neither the patients nor the health care providers knew which treatment was being given until the trials were completed. The treatment continued until the disease progressed or the side effects became too toxic.
The trial measured the duration of time in each group before the women’s tumors worsened.p>
How were the trials designed?
The safety and efficacy efficacy of KISQALI were evaluated in one randomized, double-blind, placebo-controlled, multicenter clinical trial of KISQALI plus letrozole versus placebo plus letrozole. All patients were postmenopausal women with HR-positive, HER2-negative, advanced breast cancer who received no prior therapy for advanced disease.
KISQALI was given orally at a dose of 600 mg daily for 21 consecutive days followed by 7 days off treatment to comprise a complete cycle of 28 days. Letrozole was taken once daily continuously during the 28 day cycle.
The primary efficacy outcome measure of the trial was investigator-assessed Progression-Free Survival (PEFS) using Response Evaluation Criteria in Solid Tumors (RECIST v1.1).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.