Drug Trials Snapshot: GEMTESA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the GEMTESA Package Insert for complete information.
GEMTESA (vibegron)
gem tes' ah
Urovant Sciences, Inc.
Approval date: December 23, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
GEMTESA is used treat the following symptoms due to a condition called overactive bladder (OAB):
- a strong need to urinate with leaking or wetting accidents,
- the need to urinate right away, and
- the need to urinate often.
How is this drug used?
GEMTESA is a tablet taken my mouth once a day.
What are the benefits of this drug?
After 12 weeks of treatment, patients who received GEMTESA experienced reduced number of OAB related symptoms in comparison to patients who received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the evaluated patients. The primary efficacy endpoints were change from baseline in average daily number of micturitions (urinations) and average daily number of urge urinary incontinence (UUI) episodes (need to urinate with leaking or wetting accidents) at week 12. Additional secondary endpoints included change from baseline in average daily number of “need to urinate immediately” (urgency) episodes and average volume voided per micturition.
Table 1. Mean Baseline and Change from Baseline at Week 12 for Micturition Frequency, Urge Urinary Incontinence Episodes, “Need to Urinate Immediately” (Urgency) Episodes, and Volume Voided per Micturition
|
Parameter |
GEMTESA |
Placebo |
|---|---|---|
|
Average Daily Number of Micturitions |
||
|
Baseline mean (n) |
11.3 (526) |
11.8 (520) |
|
Change from Baseline* (n) |
-1.8 (492) |
-1.3 (475) |
|
Difference from Placebo |
-0.5 |
|
|
95% Confidence Interval |
-0.8, -0.2 |
|
|
p-value |
<0.001 |
|
|
Average Daily Number of UUI Episodes |
||
|
Baseline mean (n) |
3.4 (403) |
3.5 (405) |
|
Change from Baseline* (n) |
-2.0 (383) |
-1.4 (372) |
|
Difference from Placebo |
-0.6 |
|
|
95% Confidence Interval |
-0.9, -0.3 |
|
|
p-value |
<0.0001 |
|
|
Average Daily Number of “Need to Urinate Immediately” (Urgency) Episodes |
||
|
Baseline mean (n) |
8.1 (526) |
8.1 (520) |
|
Change from Baseline□ (n) |
-2.7 (492) |
-2.0 (475) |
|
Difference from Placebo |
-0.7 |
|
|
95% Confidence Interval |
-1.1, -0.2 |
|
|
p-value |
0.002 |
|
|
Average Volume Voided (mL) per Micturition |
||
|
Baseline mean (n) |
155 (524) |
148 (514) |
|
Change from Baseline□ (n) |
23 (490) |
2 (478) |
|
Difference from Placebo |
21 |
|
|
95% Confidence Interval |
14, 28 |
|
|
p-value |
<0.0001 |
|
*Least squares mean adjusted for treatment, baseline, sex, geographical region, study visit, and study visit by treatment interaction term.
GEMTESA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The majority of patients were women, therefore the differences in how well the drug worked between men and women could not be determined.
- Race: The majority of patients were White, therefore the differences in how well the drug worked among races could not be determined.
- Age: GEMTESA worked similarly in patients younger than and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
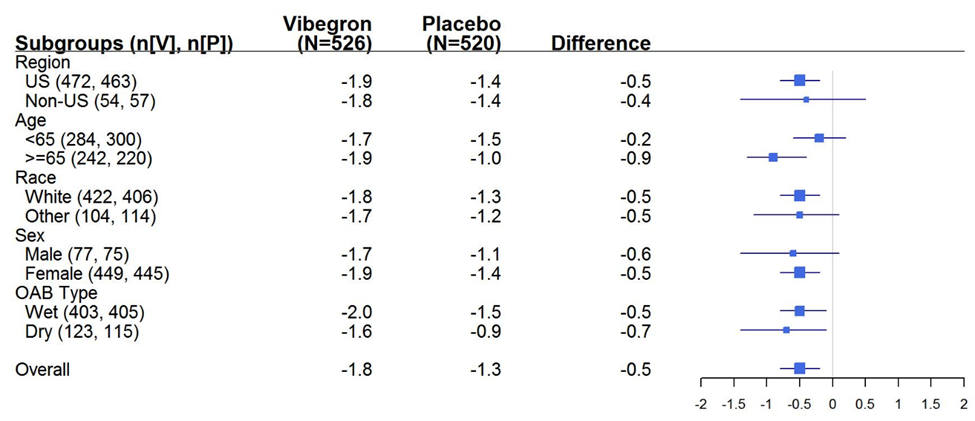
The figures below summarize efficacy results by demographic subgroups for two endpoints: average daily number of micturitions (urinations) and UUI episodes. Given the descriptive nature of these analyses and the smaller size of the subgroups, these findings should be interpreted with caution.
Figure 5. Forest Plot of GEMTESA Treatment Effect vs Placebo on Average Daily Number of Micturitions at Week 12 by Subgroup
Figure 6. Forest Plot of GEMTESA Treatment Effect vs Placebo on Average Daily Number of UUI Episodes at Week 12 by Subgroup
FDA Statistical Review
What are the possible side effects?
GEMTESA may cause serious side effects including inability to empty bladder (urinary retention).
The most common side effect of GEMTESA are headache, urinary tract infection, common cold, diarrhea, nausea, and upper respiratory tract infection.
What are the possible side effects (results of trials used to assess safety)
Presented below are common adverse reactions that were reported in the trial.
Table 2. Adverse Reactions, Exceeding Placebo Rate, Reported in ≥2% of Patients Treated with GEMTESA for up to 12 Weeks
|
|
GEMTESA |
Placebo |
|---|---|---|
|
Number of Patients |
545 |
540 |
|
Headache |
22 (4.0) |
13 (2.4) |
|
Nasopharyngitis |
15 (2.8) |
9 (1.7) |
|
Diarrhea |
12 (2.2) |
6 (1.1) |
|
Nausea |
12 (2.2) |
6 (1.1) |
|
Upper respiratory tract infection |
11 (2.0) |
4 (0.7) |
GEMTESA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects between men and women was similar.
- Race The occurrence of side effects was similar among tested races.
- Age: The occurrence of side effects was similar between patients younger than and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize the occurrence of the most common adverse reactions, by sex, race and age subgroups. Presented is the pooled safety population from two placebo-controlled Phase 3 trials where GEMTESA was used for 12 weeks at the approved dose.
Table 3. Subgroup Analysis of Common Adverse Reactions by Sex
|
|
GEMTESA |
Placebo |
|---|---|---|
|
Men |
N =82 |
N =117 |
|
Any AE |
29 (35) |
29 (25) |
|
Diarrhea |
3 (4) |
0 |
|
Headache |
2 (2) |
1 (1) |
|
Nausea |
1 (1) |
1 (1) |
|
Nasopharyngitis |
0 |
1 (1) |
|
Women |
N =463 |
N =792 |
|
Any AE |
182 (39) |
252 (32) |
|
Urinary tract infection |
26 (6) |
32 (4) |
|
Headache |
20 (4) |
12 (2) |
|
Nasopharyngitis |
15 (3) |
35 (4) |
|
Nausea |
11 (2) |
5 (1) |
|
Diarrhea |
9 (2) |
10 (1) |
Table 4. Subgroup Analysis of Common Adverse Reactions by Race
|
|
GEMTESA |
Placebo |
|---|---|---|
|
White |
N =453 |
N =418 |
|
Any AE |
174 (40) |
144 (34) |
|
Urinary tract infection |
23 (5) |
28 (7) |
|
Headache |
13 (3) |
13 (3) |
|
Nasopharyngitis |
14 (3) |
9 (2) |
|
Nausea |
11 (3) |
4 (1) |
|
Diarrhea |
12 (3) |
5 (1) |
|
Black or African American |
N =78 |
N =85 |
|
Any AE |
19 (24) |
20 (24) |
|
Urinary tract infection |
3 (4) |
4 (5) |
|
Headache |
2 (3) |
0 |
|
Upper respiratory tract infection |
2 (3) |
0 |
|
Asian |
N =28 |
N =399 |
|
Any AE |
15 (54) |
115 (29) |
|
Headache |
6 (21) |
0 |
|
Nausea |
1 (4) |
2 (1) |
|
Urinary tract infection |
1 (4) |
1 (<1) |
|
Nasopharyngitis |
0 |
27 (7) |
Table 5. Subgroup Analysis of Common Adverse Reactions by Age
|
|
GEMTESA |
Placebo |
|---|---|---|
|
< 65 Years |
N =299 |
N =550 |
|
Any AE |
101 (33.8) |
144 (26.2) |
|
Urinary tract infection |
13 (4.3) |
15 (2.7) |
|
Headache |
11 (3.7) |
8 (1.5) |
|
Nasopharyngitis |
9 (3.0) |
17 (3.1) |
|
Nausea |
7 (2.3) |
3 (0.5) |
|
Diarrhea |
6 (2.0) |
6 (1.1) |
|
≥ 65 Years |
N =246 |
N =359 |
|
Any AE |
110 (44.7) |
137 (38.2) |
|
Urinary tract infection |
14 (5.7) |
18 (5.0) |
|
Headache |
11 (4.5) |
5 (1.4) |
|
Upper respiratory tract infection |
8 (3.3) |
2 (0.6) |
|
Diarrhea |
6 (2.4) |
4 (1.1) |
|
Nasopharyngitis |
6 (2.4) |
19 (5.3) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved GEMTESA based on evidence from one clinical trial (Trial 1/ NCT03492281) of 1085 adult patients 18 to 93 years old with OAB. The trial was conducted at 199 sites in the United States, Canada, Poland Hungary, Latvia, and Lithuania.
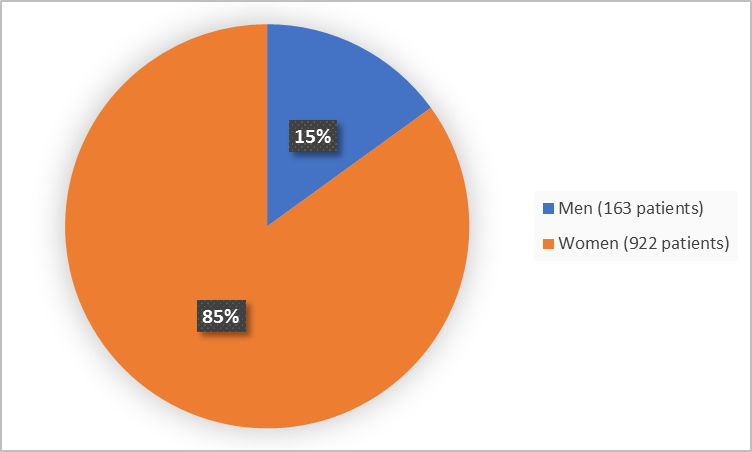
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Demographics by Sex
Adapted from FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Demographics by Race
*includes Other and American Indian or Alaska Native
Adapted from FDA Review
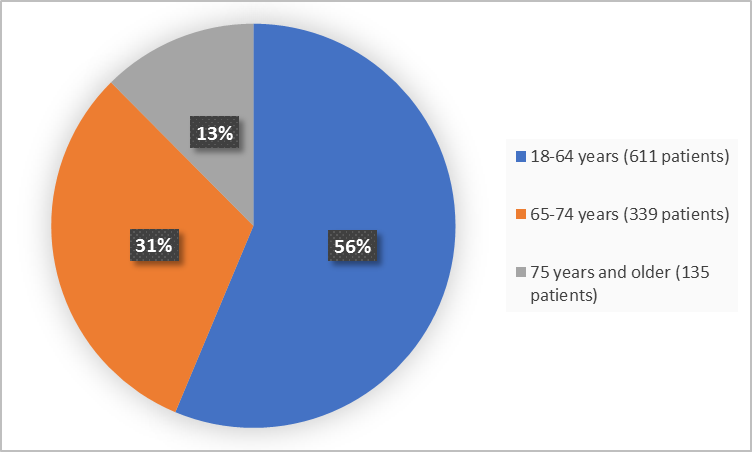
Figure 3 summarizes the percentage of patients by age in the clinical trial.
Figure 3. Demographics by Age
Adapted from FDA Review
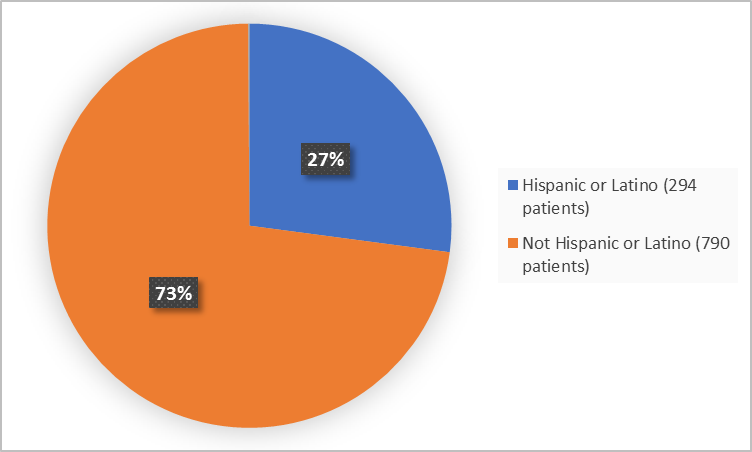
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trial.
Figure 4. Demographics by Ethnicity
Adapted from FDA Review
Who participated in the trials?
The table below summarizes demographics of the population in the Phase 3 clinical trial (Trial 1/ NCT03492281).
Table 6. Baseline Demographics (safety population)
|
|
GEMTESA |
Placebo |
TOTAL |
|---|---|---|---|
|
Sex, n (%) |
|||
|
Men |
82 (15) |
81 (15) |
163 (15) |
|
Women |
463 (85) |
459 (85) |
922 (85) |
|
Race, n (%) |
|||
|
American Indian or Alaska Native |
2 (<1) |
3 (1) |
5 (<1) |
|
Asian |
28 (5) |
30 (6) |
58 (5) |
|
Black or African American |
78 (14) |
85 (16) |
163 (15) |
|
White |
435 (80) |
418 (77) |
853 (79) |
|
Other |
2 (<1) |
4 (1) |
6 (<1) |
|
Age (years) |
|||
|
Median (Min, Max) |
63 (18,93) |
61 (19,89) |
62 (18,93) |
|
Age Group (years), n (%) |
|||
|
18 to < 65 |
299 (55) |
312 (58) |
611 (56) |
|
≥ 65 to < 75 |
171 (31) |
168 (31) |
339 (31) |
|
≥ 75 |
75 (14) |
60 (11) |
135 (13) |
|
Ethnicity |
|||
|
Hispanic or Latino |
152 (28) |
142 (26) |
294 (73) |
|
Not Hispanic or Latino |
392 (72) |
398 (74) |
790 (27) |
|
Missing |
1 (<1) |
0 |
1 (<1) |
|
Region, n (%) |
|||
|
US |
490 (90) |
483 (89) |
973 (90) |
|
Non-US |
55 (10) |
57 (11) |
112 (10) |
Adapted from FDA Review
How were the trials designed?
The benefit and side effects of GEMTESA were evaluated in one clinical trial which enrolled adult patients with OAB. Some patients were previously treated for the symptoms of OAB, and some were never treated before.
Patients received once daily treatment with either GEMTESA, placebo, or active control. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed. Patients were keeping a dairy of daily OAB symptoms.
The benefit of GEMTESA in comparison to placebo was assessed after 12 weeks by comparing the change from baseline in average daily number of voiding and average daily number of leaking accidents.
How were the trials designed?
The safety and efficacy of GEMTESA were established in one 12-week, double-blind, randomized, placebo-controlled, and active-controlled trial in patients with OAB (urge urinary incontinence, urgency, and urinary frequency). The trial population included OAB medication-naïve patients as well as patients who had received prior therapy with OAB medications. Patients were randomized to receive either GEMTESA, placebo, or active control orally, once daily for 12 weeks.
The co-primary endpoints were change from baseline in average daily number of micturitions (urinations) and average daily number of urge urinary incontinence (UUI) episodes (“need to urinate immediately” (urgency) with leaking or wetting accidents) at week 12. Additional secondary endpoints included change from baseline in average daily number of “need to urinate immediately” (urgency) episodes and average volume voided per micturition.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.