Drug Trials Snapshot: DAYVIGO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the DAYVIGO Package Insert for complete information.
DAYVIGO (lemborexant)
daye-vi'-goe
Eisai Inc.
Approval date: December 20, 2019
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
DAYVIGO is a drug for the treatment of adults with insomnia (trouble falling or staying asleep).
How is this drug used?
DAYVIGO is a tablet taken by mouth no more than once per night, immediately before going to bed.
What are the benefits of this drug?
Patients who received DAYVIGO fell asleep faster and stayed asleep longer compared to patients who received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The tables below show the efficacy assessment based on the change in sleep onset and sleep maintenance for the patients in individual trials.
Table 1. Primary and Secondary Efficacy Results for Change from Baseline in Sleep Onset and Sleep Maintenance at 6 Months in Patients with Insomnia (Trial 1)
|
Endpoint |
Treatment Group |
Number of Patients ITT |
Baseline Meana (SD) |
Month 6 LS Meana (SE) |
Treatment Effect (95% CI) |
|---|---|---|---|---|---|
|
Sleep Onset sSOL (minutes) |
DAYVIGO 5 mg* |
316 |
43.0 (31.5) |
20.0 (1.1) |
0.7 (0.6, 0.8) |
|
DAYVIGO 10 mg* |
315 |
45.0 (33.4) |
19.2 (1.1) |
0.7 (0.6, 0.8) |
|
|
Placebo |
318 |
45.0 (31.8) |
27.3 (1.4) |
(ratio vs placebo)b
|
|
|
Sleep Maintenance sSEF (%) |
DAYVIGO 5 mg* |
316 |
63.1 (18.2) |
75.9 (0.9) |
4.5 (2.2, 6.9) |
|
DAYVIGO 10 mg* |
315 |
62.0 (17.2) |
75.9 (0.9) |
4.7 (2.4, 7.0) |
|
|
Placebo |
318 |
61.3 (17.8) |
71.4 (0.8) |
(%)c |
|
|
Sleep Maintenance sWASO (minutes) |
DAYVIGO 5 mg* |
316 |
132.8 (82.5) |
87.9 (3.7) |
-17.5 (-27.3, -7.6) |
|
DAYVIGO 10 mg* |
315 |
136.8 (87.4) |
92.7 (3.7) |
-12.7 (-22.4, -3.0) |
|
|
Placebo |
318 |
132.5 (80.2) |
105.3 (3.6) |
(minutes)c |
ITT (intention to treat); sSOL (subjective sleep onset latency); SD (standard deviation); LS (least squares); SE (standard error); CI (unadjusted confidence interval); sSEF (subjective sleep efficiency); sWASO (subjective wake after sleep onset)
a For the sleep onset sSOL endpoint, the mean refers to geometric mean, which was used due to the approximately log normal distribution of the outcomes; SD for the geometric mean is calculated as GM*SD (log transformed sSOL); SE for the least squares geometric mean is calculated in the same way as the SD.
b For the sleep onset sSOL endpoint, treatment effect refers to the ratio of [Month 6 sSOL / Baseline sSOL] for DAYVIGO versus placebo, such that a smaller ratio corresponds to a greater improvement.
c Treatment effect refers to the treatment difference between DAYVIGO versus placebo, such that a larger value for sSEF and smaller value for sWASO correspond to greater improvement.
* Doses that were statistically significantly superior (p<0.05) to placebo after multiplicity adjustment.
Table 2. Primary and Secondary Efficacy Results for Change from Onset and Sleep Maintenance at 1 Month in Patients with Insomnia (Trial 2)
|
Endpoint |
Treatment Group |
Number of Patients ITT |
Baseline Meana (SD) |
Month 6 LS Meana (SE) |
Treatment Effect (95% CI) |
|---|---|---|---|---|---|
|
Sleep Onset LPS (minutes) |
DAYVIGO 5 mg* |
266 |
33.0 (27.2) |
15.5 (0.8) |
0.8 (0.7, 0.9) |
|
DAYVIGO 10 mg* |
269 |
33.3 (27.2) |
14.5 (0.7) |
0.7 (0.6, 0.8) |
|
|
Placebo |
208 |
33.6 (25.9) |
20.0 (1.1) |
(ratio vs. placebo)b |
|
|
Sleep Maintenance SEF (%) |
DAYVIGO 5 mg* |
266
|
68.4 (11.3) |
80.7 (0.5) |
7.1 (5.6, 8.5) |
|
DAYVIGO 10 mg* |
269 |
67.8 (10.8) |
82.7 (0.5) |
8.0 (6.6, 9.5) |
|
|
Placebo |
208 |
68.9 (9.6) |
74.6 (0.6) |
(%)c |
|
|
Sleep Maintenance WASO (minutes) |
DAYVIGO 5 mg* |
266 |
113.4 (39.0) |
68.3 (2.2) |
-24.0 (-30.0, -18.0) |
|
DAYVIGO 10mg* |
269 |
114.8 (40.0) |
66.9 (2.2) |
-25.3 (-31.4, -19.3) |
|
|
Placebo |
208 |
111.7 (37.2) |
92.2 (2.5) |
(minutes)c |
ITT (intention to treat); LPS (latency to persistent sleep); SD (standard deviation); LS (least squares); SE (standard error); CI (unadjusted confidence interval); SEF (sleep efficiency); WASO (wake after sleep onset)
a For the sleep onset LPS endpoint, the mean refers to geometric mean, which was used due to the approximately log normal distribution of the outcomes; SD for the geometric mean is calculated as GM*SD(log transformed LPS); SE for the least squares geometric mean is calculated in the same way as the SD.
b For the LPS endpoint, treatment effect refers to the ratio of [Day 29/30 LPS / Baseline LPS] for DAYVIGO versus placebo, such that a smaller ratio corresponds to a greater improvement.
c Treatment effect refers to the treatment difference between DAYVIGO versus placebo, such that a larger value for SEF and smaller value for WASO correspond to greater improvement.
* Doses that were statistically significantly superior (p<0.05) to placebo after multiplicity adjustment.
DAYVIGO Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: DAYVIGO worked similarly in men and women.
- Race: DAYVIGO worked similarly in tested races.
- Age: DAYVIGO worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race and age groups?
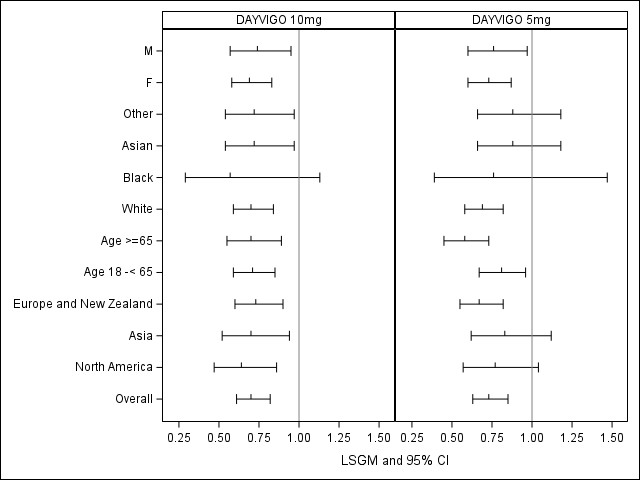
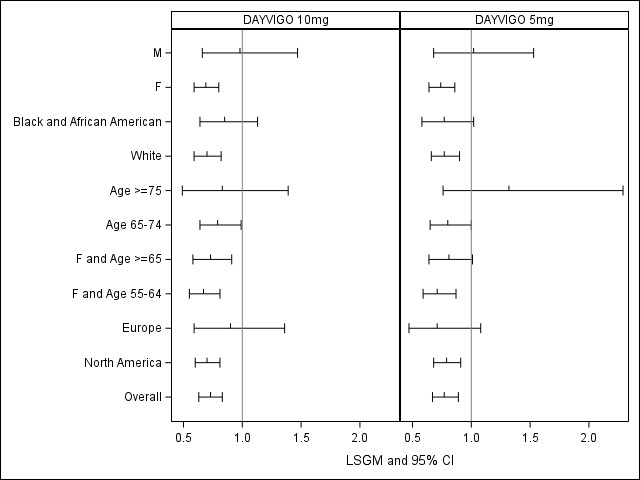
The figures below show subgroup analyses based on primary endpoints in each trial. Some subgroups are small, limiting interpretation of these findings. In subgroup analyses on two key secondary endpoints (SEF and WASO) in Trial 2 (not shown here), the subgroups generally had effects favoring DAYVIGO.
Figure 4. LSGM Treatment Ratio (Active/Placebo) with 95% CI in sSOL by Subgroup, Trial 1
LSGM= least squares geometric mean; CI=confidence interval; sSOL= subject sleep onset latency; F= female; M=male
Adapted from FDA Review
Figure 5. LSGM Treatment Ratio (Active/Placebo) with 95% CI in LPS by Subgroup, Trial 2
LSGM=least squares geometric mean; CI= confidence interval; LPS= latency to persistence sleep; F= female; M=male
Adapted from FDA Review
What are the possible side effects?
DAYVIGO may cause serious side effects including:
- decreased daytime alertness and impaired ability to operate a vehicle
- sleep paralysis (temporary inability to move or talk while going to sleep or waking up)
- hallucinations that occur while going to sleep or waking up
- complex sleep behaviors (e.g., sleep-walking, sleep-driving)
- worsening depression and suicidal thoughts.
The most common side effect of DAYVIGO is drowsiness.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes the adverse reactions that occurred during the first 30 days in the pooled population from the two clinical trials.
Table 3. Adverse Reactions Reported in ≥ 2% of DAYVIGO-Treated Patients and at a Greater Frequency than Placebo-Treated Patients During the First 30-days of Trials 1 and 2
|
Adverse Reaction |
DAYVIGO |
Placebo n=528 |
|
|
5 mg n=580 |
10 mg n=582 |
||
|
(%) |
(%) |
(%) |
|
|
Somnolence or fatigue* |
6.9 |
9.6 |
1.3 |
|
Headache |
5.9 |
4.5 |
3.4 |
|
Nightmare or abnormal dreams |
0.9 |
2.2 |
0.9 |
* Combines preferred terms somnolence, lethargy, fatigue, sluggishness
DAYVIGO Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar between men and women.
- Race: The occurrence of side effects was similar among tested races.
- Age: Drowsiness occurred more frequently in older patients who were taking the higher DAYVIGO dose. Otherwise, the occurrence of side effects was similar between patients younger and older than 65 years of age.
Were there any differences in side effects among sex, race and age groups?
The tables below summarize the main adverse events of interest, somnolence and parasomnia, by subgroups. The tables include all patients who received placebo or DAYVIGO in Trial 1, Trial 2, and the 6-month extension of Trial 1, where the all patients received DAYVIGO.
Table 4. Treatment-Emergent Adverse Reaction Somnolence by Subgroup
|
|
Placebo |
DAYVIGO 5mg |
DAYVIGO 10 mg |
||||
|---|---|---|---|---|---|---|---|
|
|
Treatment Duration (patient-years) |
Events per 100 patient-years |
Treatment Duration (patient-years) |
Events per 100 patient-years |
Treatment Duration (patient-years) |
Events per 100 patient-years |
|
|
Overall |
158.6 |
6.3 |
340.2 |
19.4 |
315.2 |
31.4 |
|
|
Sex |
Men |
48.4 |
2.1 |
108.2 |
15.7 |
93.6 |
39.5 |
|
Women |
110.2 |
8.2 |
232.0 |
21.1 |
221.5 |
28.0 |
|
|
Race |
Asian |
26.7 |
0.0 |
63.8 |
14.1 |
60.8 |
23.0 |
|
Black |
13.9 |
21.6 |
29.0 |
17.3 |
24.5 |
28.5 |
|
|
Other |
2.1 |
48.8 |
2.9 |
68.2 |
4.3 |
92.7 |
|
|
White |
115.9 |
5.2 |
244.4 |
20.5 |
225.5 |
32.8 |
|
|
Age |
less than 65 y |
110.1 |
6.4 |
239.7 |
20.0 |
222.2 |
27.5 |
|
over 65 y |
48.4 |
6.2 |
100.4 |
17.9 |
93.0 |
40.9 |
|
|
Ethnicity |
Hispanic/Latino |
16.3 |
6.2 |
24.6 |
24.4 |
20.7 |
29.0 |
|
Not Hispanic/Latino |
142.3 |
6.3 |
315.6 |
19.0 |
294.5 |
31.6 |
|
Table 5. Treatment -Emergent Adverse Reaction Parasomnia by Subgroup
|
|
Placebo |
DAYVIGO 5 mg |
DAYVIGO 10 mg |
||||
|---|---|---|---|---|---|---|---|
|
|
Treatment Duration (patient-years) |
Events per 100 patient-years |
Treatment Duration (patient-years) |
Events per 100 patient-years |
Treatment Duration (patient-years) |
Events per 100 patient-years |
|
|
Overall |
158.6 |
0.0 |
340.2 |
3.2 |
315.2 |
5.4 |
|
|
Sex |
Men |
48.4 |
0.0 |
108.2 |
2.8 |
93.6 |
4.3 |
|
Women |
110.2 |
0.0 |
232.0 |
3.4 |
221.5 |
5.9 |
|
|
Race |
Asian |
26.7 |
0.0 |
63.8 |
6.3 |
60.8 |
6.6 |
|
Black |
13.9 |
0.0 |
29.0 |
3.5 |
24.5 |
0.0 |
|
|
Other |
2.1 |
0.0 |
2.9 |
0.0 |
4.3 |
0.0 |
|
|
White |
115.9 |
0.0 |
244.4 |
2.5 |
225.5 |
5.8 |
|
|
Age |
less than 65 y |
110.1 |
0.0 |
239.7 |
3.3 |
222.2 |
5.9 |
|
over 65 y |
48.4 |
0.0 |
100.4 |
3.0 |
93.0 |
4.3 |
|
|
Ethnicity |
Hispanic/Latino |
16.3 |
0.0 |
24.6 |
0.0 |
20.7 |
4.8 |
|
Not Hispanic/Latino |
142.3 |
0.0 |
315.6 |
3.5 |
294.5 |
5.4 |
|
Adapted from FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved DAYVIGO for insomnia based primarily on evidence from two trials (Trial 1/NCT02952820 and Trial 2/NCT02783729) with a total of 1,692 patients. The trials were conducted at 164 sites in the United States, Canada, Europe, Japan, Korea, Taiwan and Mexico.
Presented below is the population that provided data for the assessment of DAYVIGO’s benefit (efficacy population).
Figure 1 below summarizes how many men and women were in the clinical trials.
Figure 1. Demographics by Sex (efficacy population)
FDA Review
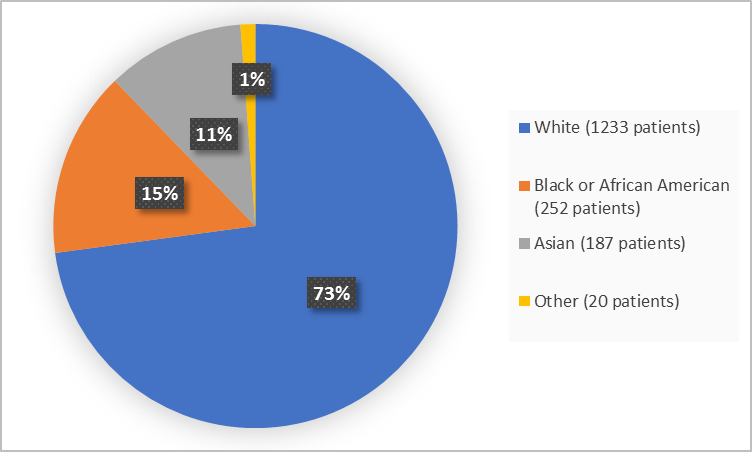
Figure 2 summarizes the percentages of patients by race in the clinical trials.
Figure 2. Demographics by Race (efficacy population)
FDA Review
Figure 3 summarizes the percentages of patients by age group in the clinical trials.
Figure 3. Demographics by Age (efficacy population)
FDA Review
Who participated in the trials?
The table below summarizes the demographics for the efficacy population.
Table 6. Demographic Information for the Efficacy Population
|
Demographic Parameters |
Trial 1 (N=949) |
Trial 2 (N=743) |
TOTAL (N=1692) |
|---|---|---|---|
|
Sex, n (%) |
|
|
|
|
Men |
302 (31.8) |
100 (13.5) |
402 (23.7) |
|
Women |
647 (68.2) |
643 (86.5) |
1290 (76.2) |
|
Race, n (%) |
|
|
|
|
White |
679 (71.5) |
554 (74.6) |
1233 (72.9) |
|
Black or African American |
76 (8.0) |
176 (23.7) |
252 (14.9) |
|
Asian |
178 (18.8) |
9 (1.2) |
187 (11.1) |
|
Other |
16 (1.7) |
4 (0.5) |
20 (1.2) |
|
Age |
|
|
|
|
Mean years (SD) |
54.5 (13.80) |
63.8 (6.69) |
58.6 (12.2) |
|
Median (years) |
55 |
63 |
60 |
|
Min, max (years) |
18, 88 |
55, 88 |
18, 88 |
|
Age Group, n (%) |
|
|
|
|
< 65 years |
687 (72.4) |
410 (55.2) |
1097 (64.8) |
|
≥ 65 years |
262 (27.6) |
333 (44.8) |
595 (35.2) |
|
Ethnicity, n(%) |
|
|
|
|
Hispanic or Latino |
72 (7.6) |
133 (17.9) |
205 (12.1) |
|
Not Hispanic or Latino |
877 (92.4) |
610 (82.1) |
1487 (87.9) |
|
Region, n (%) |
|
|
|
|
USA |
292 (30.8) |
622 (83.7) |
914 (54.0) |
|
Canada |
9 (0.9) |
15 (2) |
24 (1.4) |
|
Mexico |
1 (0.1) |
0 (0) |
1 (0.1) |
|
Europe |
468 (49.3) |
106 (14.3) |
574 (33.9) |
|
New Zealand |
15 (1.6) |
0 (0) |
15 (0.9) |
|
Asia |
164 (17.3) |
0 (0) |
164 (9.7) |
How were the trials designed?
The benefits and side effects of DAYVIGO were primarily evaluated in two clinical trials.
In Trial 1 patients received DAYVIGO at one of two different strengths (5 mg or 10 mg) or a placebo for 6 months. Neither the patients nor the healthcare providers knew which treatment was being given. Patients kept a diary where they recorded patterns of their sleep. The benefit of DAYVIGO was evaluated by comparing changes in sleep as reported by the patients, comparing the DAYVIGO and placebo groups.
In Trial 2, patients with insomnia (women 55 years and older and men 65 years and older) received DAYVIGO at one of two strengths (5 mg or 10 mg), placebo, or an approved comparator drug for 1 month. Neither the patients nor the healthcare providers knew which treatment was being given. The benefit of DAYVIGO was evaluated by comparing changes in sleep between patients given DAYVIGO and patients given placebo. Sleep patterns were measured using overnight polysomnography (PSG), a sleep laboratory test.
How were the trials designed?
The efficacy and safety of DAYVIGO were assessed in adult patients who met DSM-5 criteria for insomnia disorder. Patients were evaluated in two multicenter, randomized, double-blind, placebo-controlled trials.
In Trial 1, patients were randomized to DAYVIGO 5 mg, DAYVIGO 10 mg, or placebo once nightly for 6 months. The primary endpoint was the mean change from baseline to end of treatment at 6 months for log transformed patient-reported (subjective) sleep onset latency (sSOL), defined as the number of minutes from the time the subject attempted to sleep until they fell asleep. Sleep maintenance was measured with two secondary endpoints: subjective sleep efficiency (sSEF), the subject’s perception of the proportion of time they were asleep while in bed, and sWASO, the number of minutes awake between the onset of sleep and wake time. All endpoints were measured by a sleep diary and therefore reflected patients’ perceptions of their sleep.
In Trial 2 women 55 years and older and men 65 years and older were randomized to DAYVIGO 5 mg, DAYVIGO 10 mg, placebo, or an active comparator once nightly for one month. The primary endpoint was the mean change in log transformed latency to persistent sleep (LPS) from baseline to end of treatment (Days 29/30). LPS was defined as the number of minutes from lights off to the first 10 consecutive minutes of non-wakefulness. The secondary endpoints were the mean change from baseline to end of treatment (Days 29/30) in sleep efficiency (SEF) and wake after sleep onset (WASO). In contrast to Trial 1, all endpoints were measured using overnight polysomnography monitoring.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION