Drug Trials Snapshot: Briviact
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race, and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to BRIVIACT Prescribing Information for complete information.
BRIVIACT (brivaracetam)
briv ee akt
UCB, Inc.
Approval date: February 18, 2016
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
BRIVIACT is a drug for the treatment of specific type of seizures called partial-onset seizures in patients 16 years of age and older with epilepsy. Epilepsy is a brain disorder that causes people to have recurring seizures. BRIVIACT is to be used in combination with other anti-epileptic drugs that treat the same type of seizures.
How is this drug used?
BRIVIACT is a drug that is taken two times a day by mouth. It may be in the form of tablet or liquid. BRIVIACT may be given in the vein (intravenously) when patient is not able to take it by mouth.
What are the benefits of this drug?
Patients taking BRIVIACT along with other anti-epileptic medications had less frequent seizures than patients taking placebo with other anti-epileptic medications.
What are the benefits of this drug (results of trials used to assess efficacy)?
The tables below summarize efficacy results for the clinical trials.
Table 2. Percent Reduction in Partial-Onset Seizure Frequency over Placebo (Trials 1, 2 and 3)
| Percent Reduction Over Placebo (%) | |
|---|---|
| Trial 1a | |
| Placebo (n=100) | ------- |
| 50 mg/day (n=99) | 9.5 |
| 100 mg/day (n=100) | 17.0 |
| Trial 2a | |
| Placebo (n=96) | ------- |
| 50 mg/day (n=101) | 16.9* |
| Trial 3b | |
| Placebo (n=259) | ------ |
| 100 mg/day (n=252) | 25.2* |
| 200 mg/day (n= 249) | 25.7* |
*Statistically significant based on testing procedure with alpha = 0.05
a Based upon 7-day seizure frequency
b Based upon 28-day seizure frequency
BRIVIACT Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: BRIVIACT worked similarly in men and women.
- Race: The majority of patients in the clinical trial were white. Differences in response to BRIVIACT among races could not be determined
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in response to BRIVIACT between patients below and above 65 years of age could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The figure below summarizes efficacy results by subgroup.
Figure 4. Subgroup Analysis of Median Percent Reduction in Seizure Frequency from Baseline to Treatment Period by Sex (pooled trials)
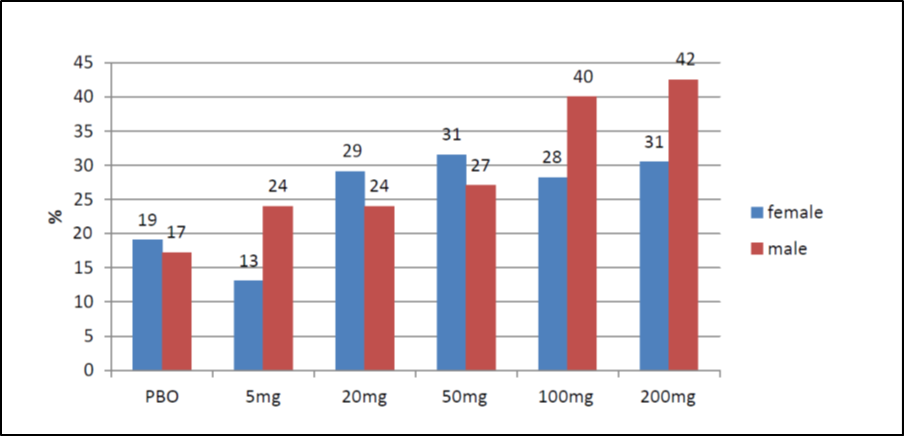
PBO=placebo
Source: FDA Clinical Review
Table 3. Subgroup Analysis of Seizure Frequency by Race (Trial 1)
| Median seizure frequency per week | Placebo N=100 | BRIVIACT 50mg N=99 | BRIVIACT 100mg N=100 |
|---|---|---|---|
| White | |||
| n | 77 | 76 | 76 |
| Baseline | 2.2 | 1.8 | 2.1 |
| Treatment period | 1.8 | 1.6 | 1.7 |
| All Other Races Combined | |||
| n | 23 | 23 | 24 |
| Baseline | 1.5 | 1.9 | 1.6 |
| Treatment period | 1.4 | 1 | 0.9 |
Source: Adapted from FDA Statistical review
Table 4. Subgroup Analysis of Seizure Frequency by Race (Trial 2)
| Median seizure frequency per week | Placebo N=96 | BRIVIACT 50mg N=101 |
|---|---|---|
| White | ||
| n | 66 | 77 |
| Baseline | 2.6 | 2.9 |
| Treatment period | 2.2 | 2 |
| All Other Races Combined | ||
| n | 30 | 24 |
| Baseline | 2.8 | 2 |
| Treatment period | 2.1 | 1.3 |
Source: Adapted from FDA Statistical review
Table 5. Subgroup Analysis of Seizure Frequency by Race (Trial 3)
| Median seizure frequency per 28 days | Placebo N=259 | BRIVIACT 100mg N=252 | BRIVIACT 200mg N=242 |
|---|---|---|---|
| White | |||
| n | 187 | 182 | 181 |
| Baseline | 10.1 | 11.8 | 9 |
| Treatment period | 8.7 | 7.3 | 5.9 |
| Asian | |||
| n | 32 | 32 | 29 |
| Baseline | 6.3 | 6.5 | 10 |
| Treatment period | 5.7 | 4.4 | 8.3 |
| All Other Races Combined | |||
| n | 40 | 38 | 29 |
| Baseline | 12.1 | 8 | 11.3 |
| Treatment period | 10.1 | 4.3 | 5.3 |
Source: Adapted from FDA Statistical review
Table 6. Subgroup Analysis of Median Seizure Frequency at Baseline and Treatment Periods by Age Groups (pooled trials)
| Placebo | BRIVIACT 50mg | BRIVIACT 100mg | BRIVIACT 200mg | |
|---|---|---|---|---|
| 18 years or less | ||||
| n | 22 | 10 | 13 | 11 |
| Baseline | 10.2 | 14.7 | 5.6 | 21.0 |
| Treatment | 13.3 | 9.0 | 2.4 | 8.7 |
| 18 to 30> | ||||
| n | 117 | 52 | 99 | 54 |
| Baseline | 11.5 | 11.2 | 11.8 | 11.2 |
| Treatment | 10.1 | 7.8 | 7.6 | 6.9 |
| 30 to 50> | ||||
| n | 237 | 102 | 175 | 134 |
| Baseline | 10.2 | 8.0 | 8.5 | 9.2 |
| Treatment | 7.7 | 5.9 | 6.5 | 6.4 |
| 50 to 65> | ||||
| n | 71 | 32 | 53 | 46 |
| Baseline | 7.5 | 7.1 | 7.9 | 7.1 |
| Treatment | 6.3 | 5.4 | 4.4 | 4.0 |
| ≥ 65 years | ||||
| n | 8 | 4 | 12 | 4 |
| Baseline | 8.5 | 15.4 | 8.0 | 6.2 |
| Treatment | 8.8 | 6.1 | 5.2 | 1.2 |
What are the possible side effects?
The most common side effects of BRIVIACT are drowsiness, dizziness, feeling tired, nausea and vomiting.
BRIVIACT may cause serious side effects including thoughts about suicide or dying, unusual changes in mood or behavior, and severe allergic reactions.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred at least 2% more frequently for BRIVIACT in pooled placebo-controlled adjunctive therapy trials in patients with partial-onset seizures.
Table 7. Adverse Reactions in Patients with Partial-Onset Seizures
| Adverse Reactions | BRIVIACT* (N=803) % | Placebo (N =459) % | ||
|---|---|---|---|---|
| Gastrointestinal disorders | ||||
| Nausea/vomiting symptoms | 5 | 3 | ||
| Constipation | 2 | 0 | ||
| Nervous system disorders | ||||
| Somnolence and sedation | 16 | 8 | ||
| Dizziness | 12 | 7 | ||
| Fatigue | 9 | 4 | ||
| Cerebellar coordination and balance** | 3 | 1 | ||
| Psychiatric disorders | ||||
| Irritability | 3 | 1 | ||
* BRIVIACT 50 mg/day, 100 mg/day, and 200 mg/day
** Cerebellar coordination and balance disturbances includes ataxia, balance disorder, coordination abnormal, and nystagmus.
Were there any differences in side effects among sex, race, and age?
- Sex: The risk of side effects was similar in men and women
- Race: The majority patients in the clinical trial were white. Differences in side effects among races could not be determined.
- Age: The majority of patients in the clinical trial were younger than 65 years of age. Differences in side effects between patients below and above 65 years of age could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes adverse reaction somnolence by subgroup.
Table 8. Subgroup Analysis of Somnolence
| Category | Placebo | BRIVIACT (≥50 mg/day) | Risk Ratio | 95% CI | |||
|---|---|---|---|---|---|---|---|
| n | total | n | total | LL | UL | ||
| Any Somnolence TEAEs * | 63 | 459 | 199 | 803 | 1.8 | 1.4 | 2.3 |
| Sex | |||||||
| Male | 37 | 230 | 84 | 398 | 1.3 | 0.9 | 1.9 |
| Female | 26 | 229 | 115 | 405 | 2.5 | 1.7 | 3.7 |
| Age | |||||||
| Adolescents (17> | 0 | 6 | 4 | 7 | 7.8 | 0.5 | 119.2 |
| Adults (17- 65 years) | 58 | 445 | 190 | 772 | 1.9 | 1.4 | 2.5 |
| Elderly (≥ 65 years) | 5 | 8 | 5 | 24 | 0.3 | 0.1 | 0.9 |
| Race | |||||||
| White | 47 | 333 | 161 | 594 | 1.9 | 1.4 | 2.6 |
| Asian | 6 | 56 | 17 | 112 | 1.4 | 0.6 | 3.4 |
| Black | 4 | 15 | 3 | 17 | 0.7 | 0.2 | 2.5 |
| Other | 6 | 52 | 17 | 75 | 2.0 | 0.8 | 4.6 |
| Missing | 0 | 3 | 1 | 5 | 1.9 | 0.1 | 34.9 |
CI=confidence interval, LL-lower limit, UL=upper limit
*Somnolence TEAEs includes somnolence, fatigue, asthenia, hypersomnia, sedation, lethargy, and malaise
Source: Adapted from FDA Clinical Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved BRIVIACT based on evidence from three clinical trials of 1558 patients with partial-onset seizures. The trials were conducted in the USA, Canada, Europe, Latin America, Asia and Australia.
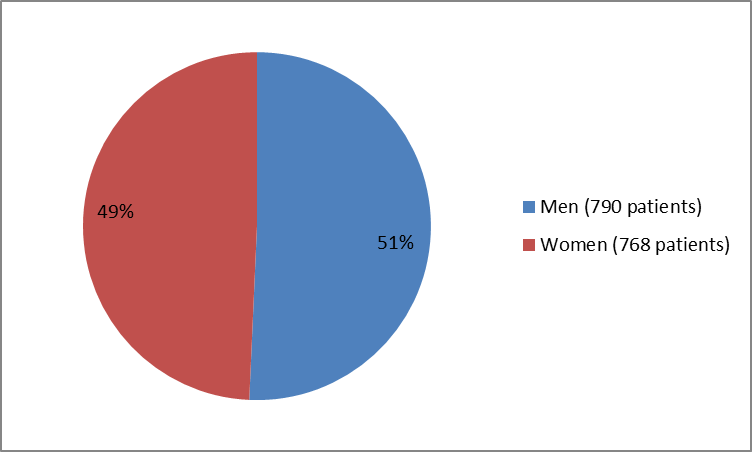
The figure below summarizes how many men and women were in the clinical trials.
Figure 1. Baseline Demographics by Sex
Clinical Trial Data
Figure 2 and Table 1 below summarize the percentage of patients by race enrolled in the clinical trials.
Figure 2. Baseline Demographics by Race
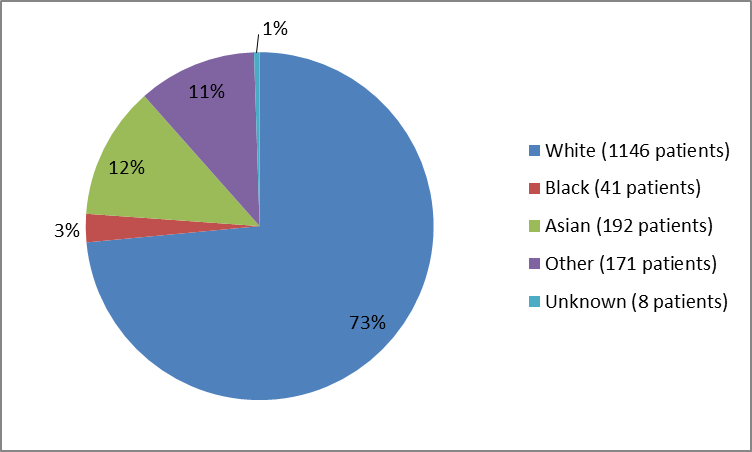
Table 1. Baseline Demographics by Race
| Race | Number of Patients | Percentage |
|---|---|---|
| White | 1146 | 73% |
| Black or African American | 41 | 3% |
| Asian | 192 | 12% |
| Other | 172 | 11% |
| Unknown | 8 | 1% |
Clinical Trial Data
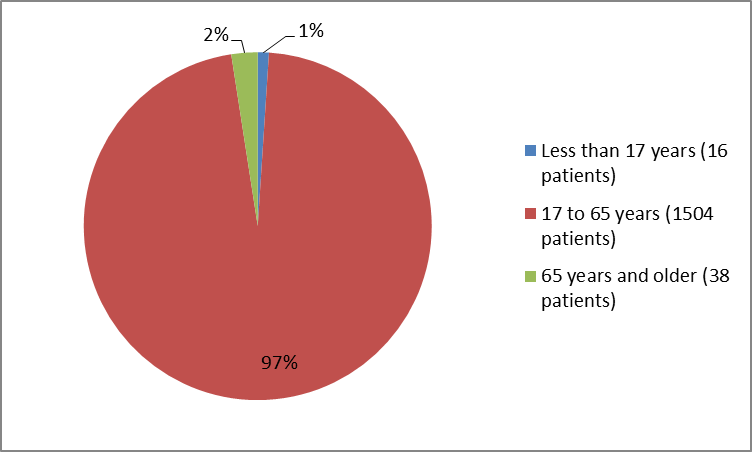
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trials.
Figure 3. Baseline Demographics by Age
Who participated in the trials?
The table below summarizes demographics of patients in the clinical trials.
Table 9. Baseline Demographics of Patients in the Clinical Trials
| Parameter | Statistic | Placebo (N=459) | BRIVIACT dose/day | BRIVIACT Overall (N=1099) | All subjects (N=1558) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5mg (N=97) | 20mg (N=199) | 50mg (N=200) | 100mg (N=353) | 200mg (N=250) | |||||
| Age (years) | n | 459 | 97 | 199 | 200 | 353 | 250 | 1099 | 1558 |
| Mean (SD) | 38.2 (12.7) | 38.3 (11.6) | 35.9 (12.9) | 38.3 (12.9) | 38.6 (13.3) | 39.8 (12.8) | 38.3 (12.9) | 38.3 (12.9) | |
| Median | 37.0 | 38.0 | 35.0 | 38.0 | 38.0 | 40.0 | 38.0 | 38.0 | |
| Min-Max | 16-77 | 18-69 | 14-69 | 16-70 | 16-80 | 16-73 | 14-80 | 14-80 | |
| Age group (years) | |||||||||
| > | n (%) | 6 (1.3) | 0 | 3 (1.5) | 2 (1.0) | 2 (0.6) | 3 (1.2) | 10 (0.9) | 16 (1.0) |
| 17-> | n (%) | 445 (96.9) | 95 (97.9) | 192 (96.5) | 194 (97.0) | 337 (95.5) | 241 (96.4) | 1059 (96.4) | 1504 (96.5) |
| ≥65 | n (%) | 8 (1.7) | 2 (2.1) | 4 (2.0) | 4 (2.0) | 14 (4.0) | 6 (2.4) | 30 (2.7) | 38 (2.4) |
| Sex | |||||||||
| Male | n (%) | 230 (50.1) | 49 (50.5) | 113 (56.8) | 105 (52.5) | 160 (45.3) | 133 (53.2) | 560 (51.0) | 790 (50.7) |
| Female | n (%) | 229 (49.9) | 48 (49.5) | 86 (43.2) | 95 (47.5) | 193 (54.7) | 117 (46.8) | 539 (49.0) | 768 (49.3) |
| Race | |||||||||
| White | n (%) | 333 (72.5) | 74 (76.3) | 145 (72.9) | 154 (77.0) | 258 (73.1) | 182 (72.8) | 813 (74.0) | 1146 (73.6) |
| Black | n (%) | 15 (3.3) | 4 (4.1) | 5 (2.5) | 2 (1.0) | 8 (2.3) | 7 (2.8) | 26 (2.4) | 41 (2.6) |
| Asian | n (%) | 56 (12.2) | 0 | 24 (12.1) | 27 (13.5) | 56 (15.9) | 29 (11.6) | 136 (12.4) | 192 (12.3) |
| Other | n (%) | 52 (11.3) | 19 (19.6) | 25 (12.6) | 17 (8.5) | 29 (8.2) | 29 (11.6) | 119 (10.8) | 171 (11.0) |
| Missing | n (%) | 3 (0.7) | 0 | 0 | 0 | 2 (0.6) | 3 (1.2) | 5 (0.5) | 8 (0.5) |
Clinical Trial Data
How were the trials designed?
The benefit and side effects of BRIVIACT were evaluated in three clinical trials of patients with partial-onset seizures. All patients were taking their usual treatments for the seizures. In addition, patients received new treatment with either BRIVIACT or placebo for 12 weeks. Neither the patients nor the health care providers knew which new treatment was being given until after the trial was completed.
The benefit of BRIVIACT was evaluated by measuring the difference in number of seizures for the patients receiving BRIVIACT in comparison to the patients who took placebo. In two trials that measurement was taken during 7 days after treatment and in one trial during 28 days after the treatment.
How were the trials designed?
The safety and efficacy of BRIVIACT were established in in 3 fixed-dose, randomized, double-blind, placebo-controlled, multicenter trials. Patients had to have partial-onset seizures that were not adequately controlled with 1 to 2 concomitant antiepileptic drugs.
The trials had an 8-week baseline period followed by a 12-week treatment period.
The efficacy outcome measure in Trials 1 and 2 was the percent reduction in 7-day partial-onset seizure frequency over placebo, while the primary outcome for Trial 3 was the percent reduction in 28-day partial-onset seizure frequency over placebo.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.