Drug Trial Snapshots: MONJUVI
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the MONJUVI Prescribing Information for complete information.
MONJUVI (tafasitamab-cxix)
(mon-JOO-vee)
MorphoSys US Inc.
Approval date: July 31, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
MONJUVI is used to treat adults with diffuse large B-cell lymphoma (DLBCL) whose disease has come back or has not improved after previous treatments. It is to be used when patients cannot receive a stem cell transplant.
DLBCL is a fast-growing cancer of the lymph system, which is part of the body’s immune system.
How is this drug used?
MONJUVI is given by a healthcare provider directly into the bloodstream through a needle in the vein. This is known as an intravenous, or IV infusion. MONJUVI is given according to a specific cycle schedule and together with lenalidomide (a type of chemotherapy).
What are the benefits of this drug?
In a clinical trial, of 71 patients with DLBCL assigned to get MONJUVI plus lenalidomide, 55% had complete or partial shrinkage of their tumors that lasted about 22 months.
MONJUVI was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
There are ongoing clinical trials to confirm the clinical benefit of MONJUVI.
What are the benefits of this drug (results of trials used to assess efficacy)?
Presented below is the best overall response rate defined as the proportion of complete and partial responders and duration of response (DOR) as assessed by an Independent Review Committee (IRC) according to the International Working Group Response Criteria.
Table 1. Efficacy Results in Trial 1
|
Efficacy Parameter |
N = 71 |
|---|---|
|
Best overall response rate, n (%) |
39 (55%) |
|
Duration of Response |
|
a Kaplan Meier estimates
MONJUVI Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
The trial that looked at the benefit of MONJUVI was too small to determine if there were any differences in sex, race and age subgroups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by demographic subgroups. Because of the small sample sizes, these exploratory analyses should be interpreted with caution.
Table 2. Subgroup Analyses of ORR
|
Responders/Total |
ORR (95% CI) |
|
|---|---|---|
|
Overall |
39/71 |
55% (42.7%, 66.8%) |
|
Sex |
||
|
Women |
16/32 |
50% (31.9%, 68.1%) |
|
Men |
23/39 |
59% (42.1%, 74.4%) |
|
Race |
||
|
White |
37/62 |
60% (46.4%, 71.9%) |
|
Missing |
2/6 |
33% (4.3%, 77.7%) |
|
Age Group |
||
|
<= 70 years |
19/32 |
59% (40.6%, 76.3%) |
|
> 70 years |
20/39 |
51% (34.8%, 67.6%) |
FDA Review
What are the possible side effects?
MONJUVI may cause serious side effects including infusion related reactions, bone marrow suppression, infections, and harm to an unborn baby.
The most common side effects of MONJUVI are low blood cell counts, fatigue, diarrhea, cough, fever, limb swelling, upper respiratory infection, and decreased appetite.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions.
Table 3. Adverse Reactions Occurring in >10% of Patients with Relapsed or Refractory DLBCL
|
Adverse Reaction* |
MONJUVI |
|
|---|---|---|
|
All Grades |
Grade 3 or 4 |
|
|
Blood and lymphatic system disorders |
||
|
Neutropenia |
51 |
49 |
|
Anemia |
36 |
7 |
|
Thrombocytopenia |
31 |
17 |
|
Febrile neutropenia |
12 |
12 |
|
General disorders and administration site conditions |
||
|
Fatigue* |
38 |
3.7 |
|
Pyrexia |
24 |
1.2 |
|
Peripheral edema |
24 |
0 |
|
Gastrointestinal disorders |
||
|
Diarrhea |
36 |
1.2 |
|
Constipation |
17 |
0 |
|
Nausea |
15 |
0 |
|
Vomiting |
15 |
0 |
|
Respiratory, thoracic and mediastinal disorders |
||
|
Cough |
26 |
1.2 |
|
Dyspnea |
12 |
1.2 |
|
Infections |
||
|
Respiratory tract infection+ |
24 |
4.9 |
|
Urinary tract infection† |
17 |
4.9 |
|
Bronchitis |
16 |
1.2 |
|
Metabolism and nutrition disorders |
||
|
Decreased appetite |
22 |
0 |
|
Hypokalemia |
19 |
6 |
|
Musculoskeletal and connective tissue disorders |
||
|
Back pain |
19 |
2.5 |
|
Muscle spasms |
15 |
0 |
*Fatigue includes asthenia and fatigue
+ Respiratory tract infection includes:lower respiratory tract infection, upper respiratory tract infection, respiratory tract infection
† includes: urinary tract infection, Escherichia urinary tract infection, urinary tract infection bacterial, urinary tract infection enterococcal
MONJUVI Prescribing Information
Were there any differences in side effects among sex, race and age?
The trial that looked at the side effects of MONJUVI was too small to determine differences in sex, race and age subgroups.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes selected adverse reactions by sex, and age subgroup. Because of the small sample sizes, these exploratory analyses should be interpreted with caution.
Table 4. Subgroup Analyses of Selected Adverse Reactions
|
Adverse Reactions |
Overall |
Sex |
Age |
||
|---|---|---|---|---|---|
|
Women |
Men |
<65 years |
≥65 years |
||
|
Blood and Lymphatic System Disorders |
65.4 |
70.3 |
61.4 |
65.2 |
65.5 |
|
Infections and Infestations |
72.8 |
75.7 |
70.5 |
73.9 |
72.4 |
FDA Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved MONJUVI based primarily on evidence from one clinical trial (NCT02399085) of 81 patients 42 to 86 years old. Patients who participated in the trial had lymphoma that came back or did not improved after prior treatments. The trial was conducted at 35 sites in the United States and Europe.
Presented below is the trial population that provided data to assess the side effects of MONJUVI (called the safety population).
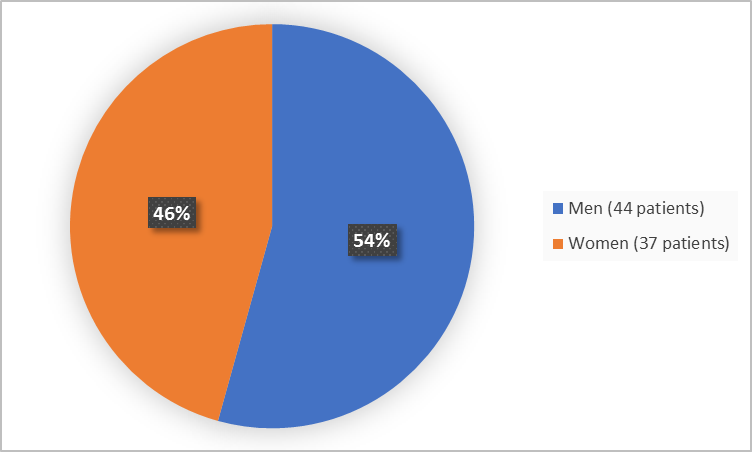
Figure 1 summarizes how many men and women participated in the trial.
Figure 1. Demographics by Sex (Safety Population)
FDA Review
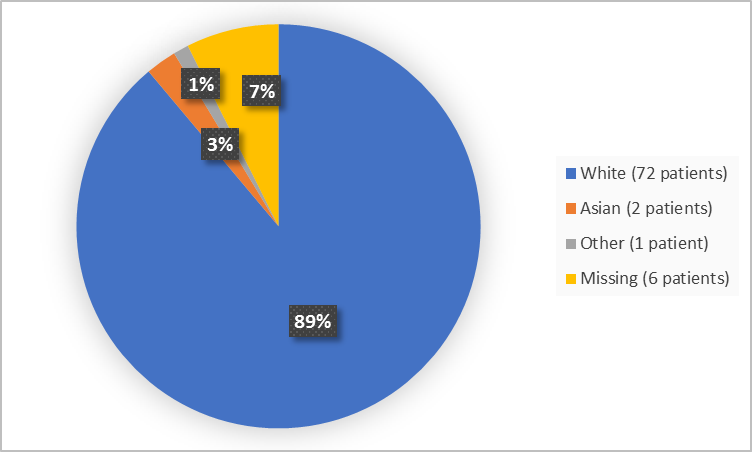
Figure 2. summarizes trial patients by race.
Figure 2. Demographics by Race (Safety Population)
FDA Review
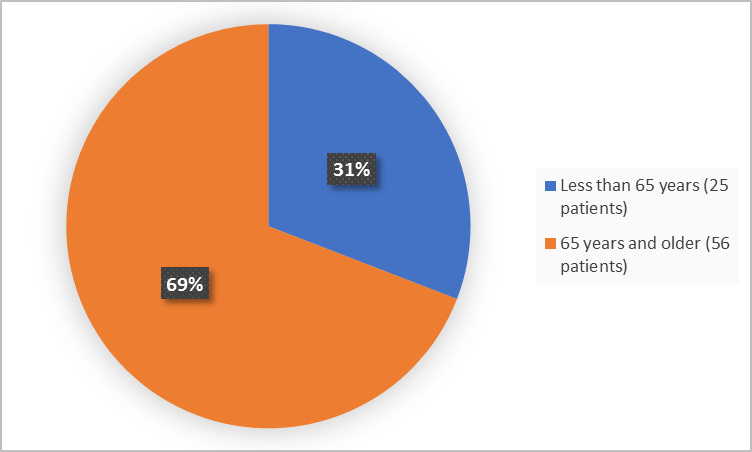
Figure 3 summarizes patients who participated in the trial by age.
Figure 3. Demographics by Age (Safety Population)
FDA Review
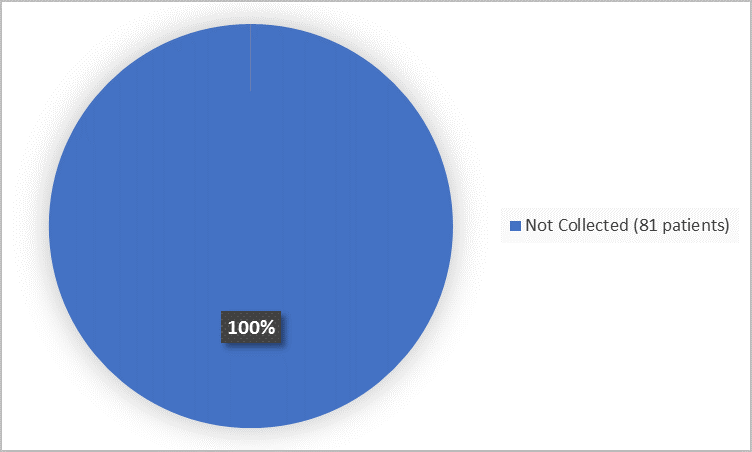
Figure 4 summarizes patients who participated in the trial by ethnicity.
Figure 4. Demographics by Ethnicity (Safety Population)
FDA Review
Who participated in the trials?
The table below summarizes trial demographics.
Table 5. Demographics of Patients (Safety Population)
|
Demographic Category |
MONJUVI |
|---|---|
|
Sex, n (%) |
|
|
Men |
44 (54.3) |
|
Women |
37 (45.7) |
|
Race, n (%) |
|
|
White |
72 (88.9) |
|
Asian |
2 (2.5) |
|
Other |
1 (1.2) |
|
Missing/Not available |
6 (7.4) |
|
Age (years) |
|
|
Median |
72.0 |
|
Min, Max |
41, 86 |
|
Age Groups, n (%) |
|
|
≤65 years |
25 (30.9) |
|
>65 years |
56 (69.1) |
|
≤75 years |
58 (71.6) |
|
>75 years |
23 (28.4) |
|
Ethnicity, n (%) |
|
|
Not Collected |
81 (100) |
|
Region, n (%) |
|
|
USA |
6 (7.4) |
|
Europe |
75 (92.6) |
Adapted from FDA review
How were the trials designed?
There was one trial that evaluated the benefit and side effects of MONJUVI in patients with lymphoma whose disease had returned or had not improved after previous treatment.
At first, patients received MONJUVI in combination with lenalidomide and later MONJUVI alone following a specific schedule during each 28-day treatment cycle. Treatment continued until disease progression or unacceptable side effects. Both patients and health care providers knew which treatment had been given.
The benefit of MONJUVI was evaluated by measuring how many patients had a complete or partial tumor shrinkage and how long that response lasted (called best overall response rate).
How were the trials designed?
The safety and efficacy of MONJUVI were established in an open label, multicenter trial in adult patients with relapsed or refractory DLBCL after 1 to 3 prior systemic therapies. Enrolled patients were not candidates for high dose chemotherapy followed by autologous stem cell transplantation.
Patients received MONJUVI 12 mg/kg intravenously in combination with lenalidomide (25 mg orally on Days 1 to 21 of each 28-day cycle) for maximum of 12 cycles, followed by MONJUVI as a monotherapy until disease progression or unacceptable toxicity.
Efficacy was established based on best overall response rate, defined as the proportion of complete and partial responders, and duration of response as determined by an IRC using the International Working Group Response Criteria.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.