Drug Trial Snapshot: ANNOVERA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the risks and benefits of a drug. Refer to the ANNOVERA Package Insert for complete information.
ANNOVERA (segesterone acetate and ethinyl estradiol vaginal system)
ann-o-VER-ah
The Population Council, Inc.
Approval date: August 10, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
ANNOVERA is a birth control system used to prevent pregnancy in women. This system consists of a vaginal ring and two hormones.
How is this drug used?
ANNOVERA is inserted into the vagina and stays there for 3 weeks. After 3 weeks, it is removed and stays out of the vagina for one week. This schedule is repeated every 4 weeks for one year. One system (vaginal ring) is used for one year (13 cycles).
What are the benefits of this drug?
ANNOVERA prevents pregnancy, however, about 2 to 4 out of 100 women may get pregnant during the first year they use ANNOVERA.
What are the benefits of this drug (results of trials used to assess efficacy)?
Efficacy results are summarized below for patients in Trials 1 and 2. The primary outcome was the Pearl Index (PI) defined as the number of pregnancies occurring per 100 woman years (where a woman year is defined as 13 menstrual cycles).
Based on pooled data from Trials 1 and 2, 2111 females < 35 years of age completed 17427 evaluable 28-day cycles (cycles in which no back-up contraception was used). The pooled pregnancy rate, evaluated by the Pearl Index (PI), was 2.98 (95% Confidence Interval [2.13, 4.06]) per woman-years of ANNOVERA use. ANNOVERA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: All patients in the trials were women.
- Race: ANNOVERA prevents pregnancy in women of all races. However, a higher pregnancy rate was observed in the first year of ANNOVERA use among women who were not White.
- Age: All women in the trials were between 18 and 41 years of age; therefore, differences in response among age groups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by race in patients 35 years or younger.
Table 2. Pearl Index for Patients ≤ 35 Years of Age by Race
|
Subgroup |
N |
Number of pregnancies |
Number of evaluable cycles |
Pearl Index using evaluable cycles |
|---|---|---|---|---|
|
Race |
||||
|
White |
1511 |
18 |
13036 |
1.80 (1.06, 2.84) |
|
Black or African American |
296 |
12 |
1867 |
8.36 (4.32, 14.60) |
|
Other Races* |
304 |
10 |
2524 |
5.15 (2.47, 9.47) |
* includes Asian, American Indian / Alaska Native, Native Hawaiian / Pacific Islander, or multi-races.
FDA Review
What are the possible side effects?
Women over 35 years old who smoke should not use ANNOVERA because of serious side effects including death from heart attack, blood clots or stroke.
ANNOVERA may cause serious side effects including blood clots, heart attack, stroke, abnormal liver tests and high blood pressure.
The most common side effects of ANNOVERA are headache (including migraine), nausea, vomiting, vaginal yeast infections, abdominal pain, painful menstrual periods, and vaginal discharge.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in the safety population.
Table 3. Adverse Reactions Reported by ≥ 5% of ANNOVERA-treated Patients
|
Adverse Reactions |
% |
|---|---|
|
Headache, including migraine |
38.6 |
|
Nausea/vomiting |
25.0 |
|
Vulvovaginal mycotic infection/vaginal candidiasis |
14.5 |
|
Abdominal pain/lower/upper |
13.3 |
|
Dysmenorrhea |
12.5 |
|
Vaginal discharge |
11.8 |
|
Urinary tract infection (UTI)/cystitis/ pyelonephritis / genitourinary tract infection |
10.0 |
|
Breast pain/tenderness/discomfort |
9.5 |
|
Metrorrhagia/menstrual disorder |
7.5 |
|
Diarrhea |
7.2 |
|
Genital pruritus |
5.5 |
ANNOVERA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: All patients were women.
- Race: The occurrence of side effects was similar among races.
- Age: All women in the trials were between 18 and 41 years of age; therefore, differences in side effects among age groups could not be determined.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of the most common adverse reaction, headache (including migraine) by subgroup.
Table 4. Pooled Subgroup Analysis of Headache (Trials 1, 2 and 3)
|
Demographic Characteristic |
ANNOVERA |
|---|---|
|
Race, n/N (%) |
|
|
White |
674/1627 (41) |
|
Black or African American |
109/323 (34) |
|
Other |
145/358 (41) |
|
Age Group, n/N (%) |
|
|
35 years |
862/2146 (40) |
|
> 35 years |
66/162 (41) |
Clinical Trial Data
WHO WAS IN THE STUDIES?
Who participated in the clinical trials?
The FDA approved ANNOVERA based on evidence from 3 clinical trials (Trial 1/ NCT0455156/, Trial 2/NCT00263342, and Trial 3/300PK) of 2308 female patients who desired a method to prevent pregnancy. The trials were conducted at 30 sites in Australia, Dominican Republic, Europe, Latin America, and the United States.
Figure 1 summarizes how many women were in the clinical trials used to evaluate safety.
Figure 1. Baseline Demographics by Sex
FDA Review
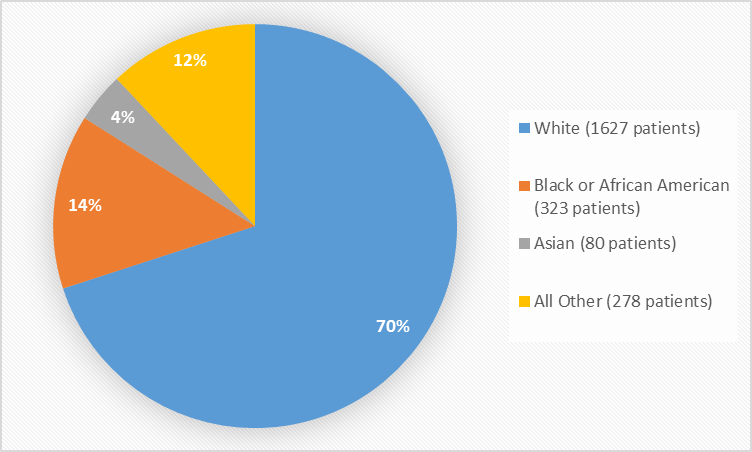
Figure 2 summarizes the percentage of patients by race in the clinical trials used to evaluate safety.
Figure 2. Baseline Demographics by Race
FDA Review
Table 1. Demographics of Safety Trials by Race
|
Race |
Number of Patients |
Percentage of Patients |
|---|---|---|
|
White |
1627 |
70% |
|
Black or African American |
323 |
14% |
|
Asian |
80 |
4% |
|
All Other* |
278 |
12% |
*Includes 22 American Indian or Native Alaskan, 5 Native Hawaiian or Pacific Islander, and 251 Other
FDA Review
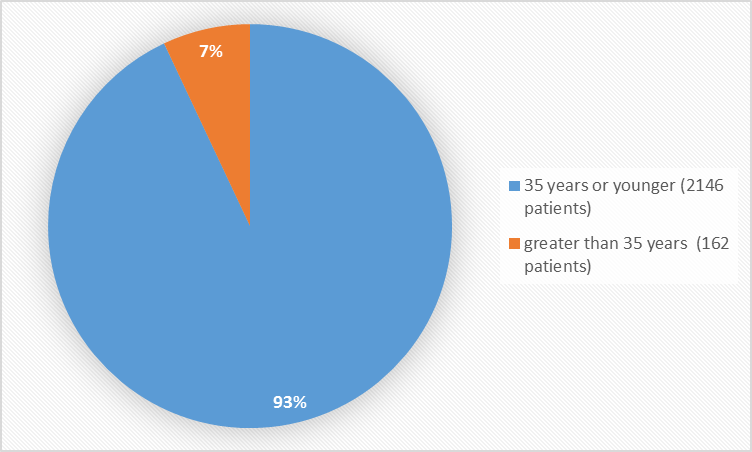
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trials used to evaluate safety.
Figure 3. Baseline Demographics by Age
FDA Review
The table below summarizes demographics of all patients in the combined clinical trials.
Table 5. Demographic Characteristics for Trials 1, 2, and 3
|
Demographic Characteristic |
ANNOVERA |
||
|---|---|---|---|
|
Sex, n (%) |
|||
|
Female |
2308 (100) |
||
|
Race, n (%) |
|||
|
White |
1627 (70) |
||
|
Black or African American |
323 (14) |
||
|
Asian |
80 (4) |
||
|
American Indian or Native Alaskan |
22 (1) |
||
|
Native Hawaiian or Pacific Islander |
5 (<> |
||
|
Other |
251 (11) |
||
|
Age Group (years) |
|||
|
35 |
2146 (93) |
||
|
> 35 |
162 (7) |
||
|
Age (years) |
|||
|
Mean (SD) |
26.7 (5.14) |
||
|
Median |
25.9 |
||
|
Min – Max |
18 - 41 |
||
|
Ethnicity |
|||
|
Hispanic |
690 (30) |
||
|
Non-Hispanic |
1618 (70) |
||
|
Region |
|||
|
United States |
1536 (67) |
||
|
Other |
772 (33) |
||
FDA Review
How were the trials designed?
The benefit and side effects of ANNOVERA were evaluated in three clinical trials. Trials 1 and 2 enrolled sexually-active women aged 18 to 40 years with regular menstrual cycles. All women desired contraception to prevent pregnancy. All women inserted ANNOVERA into the vagina for 21 days followed by removal for 7 days. This schedule was repeated every 4 weeks for up to 13 menstrual cycles. ANNOVERA was the only method of contraception. Urine pregnancy tests were performed at each visit. The benefit of ANNOVERA was assessed by calculating the number of pregnancies occurring per 100 woman years (where a woman year is defined as 13 menstrual cycles).
Trial 3 evaluated the ability of ANNOVERA to prevent release of an egg from the ovary (ovulation). Sexually-active women aged 18 to 38 years with regular menstrual cycles were enrolled. All women inserted ANNOVERA into the vagina for 21 days followed by removal for 7 days. This schedule was repeated every 4 weeks for up to 13 menstrual cycles. Patients in Trial 3 were primarily evaluated for side effects.
How were the trials designed?
The safety and efficacy of ANNOVERA were established in 3 open-label, single-arm trials.
Trials 1 and 2 enrolled sexually active women with regular menstrual cycles. Women were 18 to 40 years of age. Trial 1 was conducted solely at sites in the United States. Trial 2 was conducted at sites world-wide and include United States sites. All women inserted ANNOVERA into the vagina for 21 days followed by removal for 7 days. This schedule was repeated every 4 weeks for up to 13 menstrual cycles. Urine pregnancy tests were performed at each visit.
The primary endpoint was the Pearl Index (PI) defined as the number of pregnancies occurring per 100 woman years (where a woman year is defined as 13 menstrual cycles). The PI was calculated for women 35 years of age or younger, and derived from all cycles in which no back-up contraception was used.
Trial 3 was a Pharmacokinetic/Pharmacodynamic (PK/PD) trial which evaluated ovulation suppression in women aged 18 to 38 years with regular menstrual cycles. All women inserted ANNOVERA into the vagina for 21 days followed by removal for 7 days. This schedule was repeated every 4 weeks for up to 13 menstrual cycles. Estrogen and progesterone levels were measured twice a week during Cycles 1, 3 and 13. Patients were primarily evaluated for adverse events.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.