Office of Prescription Drug Promotion (OPDP) Metrics
This page tracks certain key metrics regarding prescription drug promotional communications. OPDP tracks the number and percentage of core launch reviews, or reviews of the initial promotional materials that companies disseminate to healthcare professionals and consumers after FDA approves a new drug (or approves a new use for a drug), completed within 45 days for each month. This page also displays the percentage of complaints regarding potentially false or misleading promotion, which may be received from health care professionals, consumers, drug sponsors, or law firms, that OPDP preliminarily reviews and acknowledges within 30 days of receipt. Finally, OPDP also tracks the number of required submissions of promotional communications to FDA on Form 2253 and the total number of submitted materials, as a submission may contain more than one promotional communication.
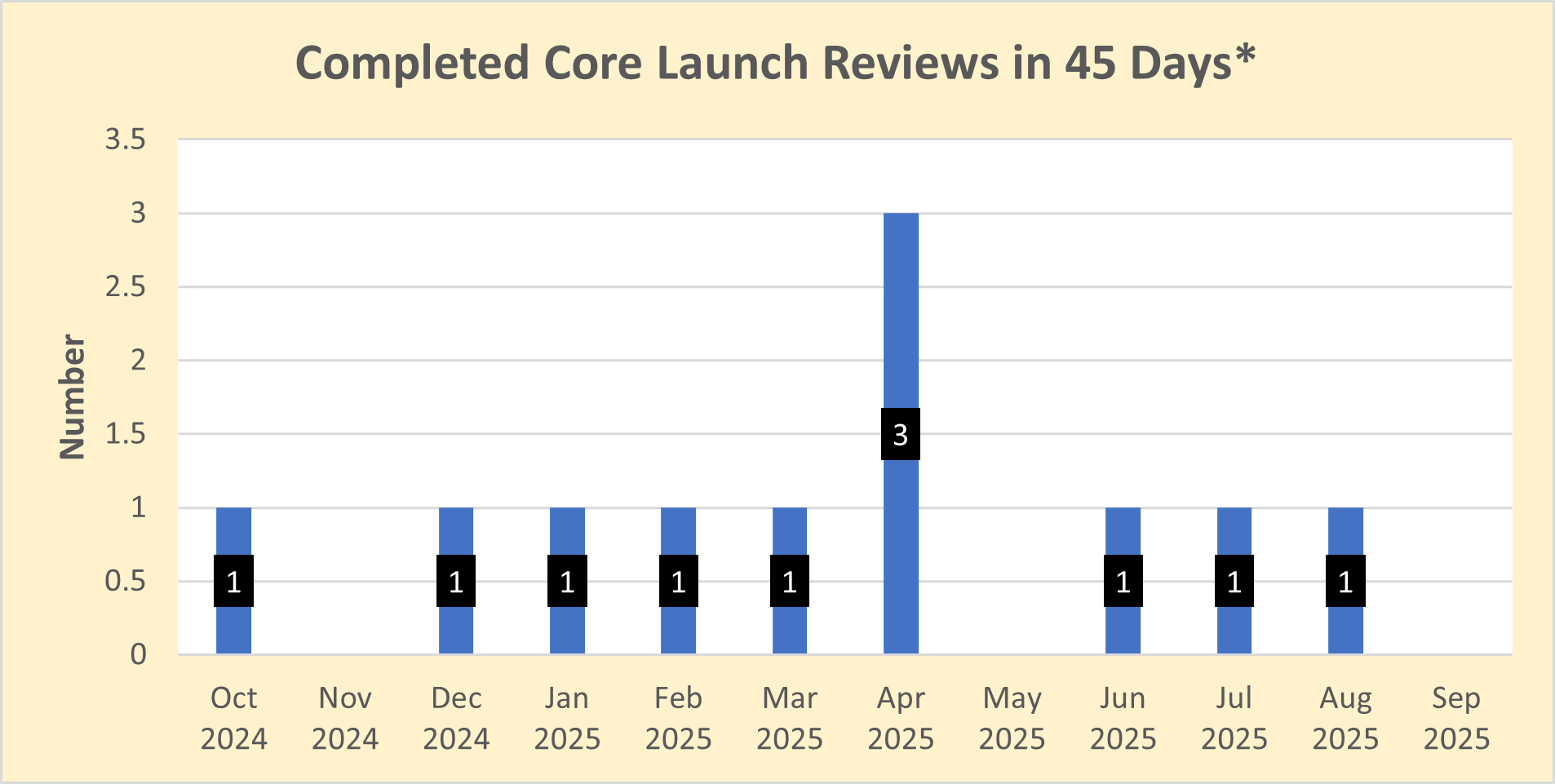
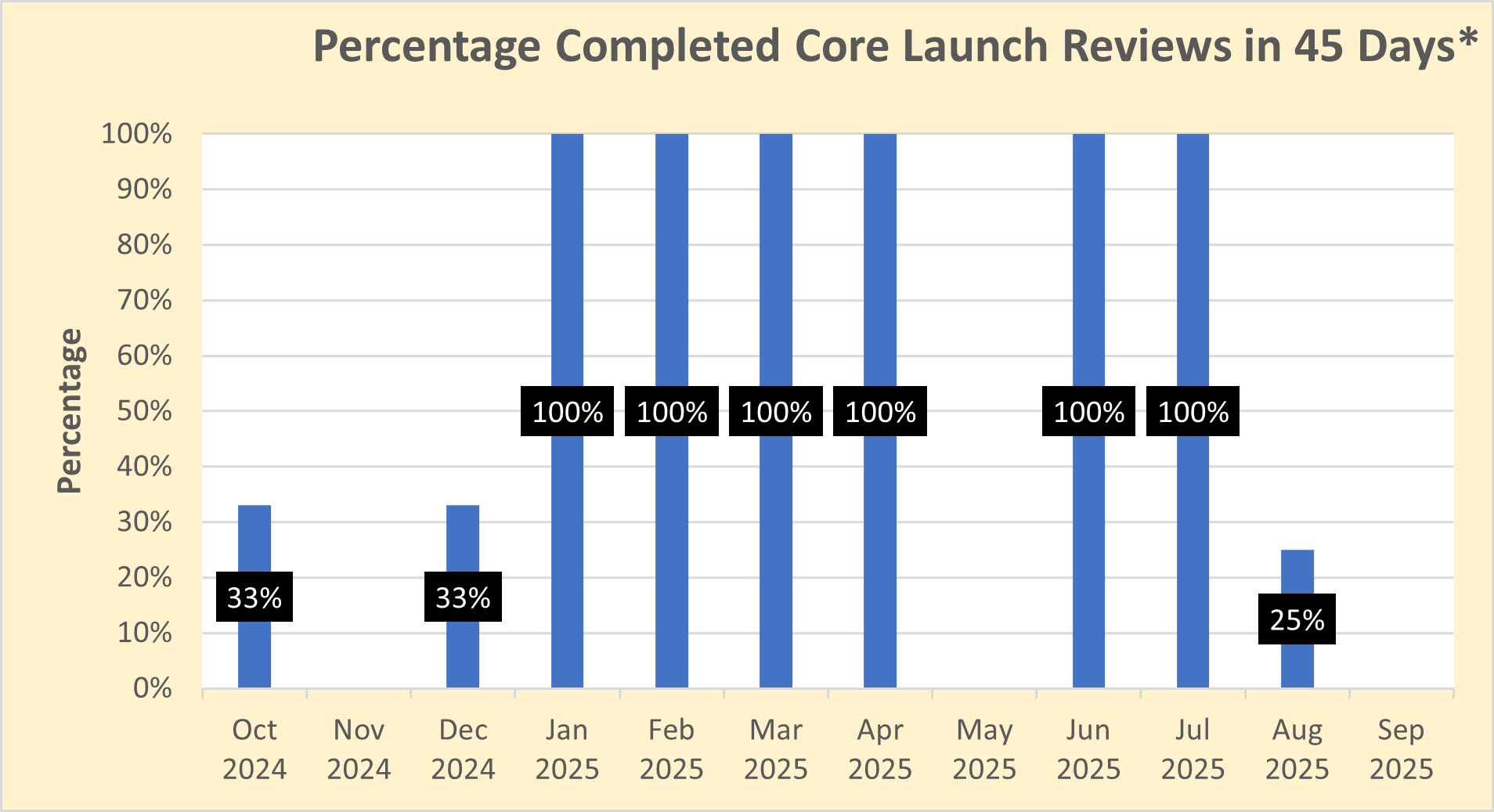
Number of core initial promotional campaign material reviews completed in the month
Dictionary: “Launch” materials are the initial promotional materials that companies disseminate to healthcare professionals and consumers after FDA approves a new drug (or approves a new use for a drug). FDA believes it is critically important to ensure that the promotional messages companies use to introduce new drugs are accurate and balanced as these messages form the public’s first impression of new drugs. The “core” launch materials are those that contain the key messages that will be used throughout the launch campaign, as well as representative examples of other important disclosures, such as risk information, for the advertised product. By reviewing and providing advisory comments on core launch campaigns in a timely manner, OPDP can positively influence the initial marketing messages that companies disseminate about their new products and help ensure that the public receives accurate and balanced information about new prescription drugs. Because this is a program measure (i.e., a measure that focuses on the OPDP program area), the time required for consultation outside of OPDP (if any) on launch campaigns is being subtracted out of the overall review time. As a result, the measure is reflective of the time it takes to review these materials within OPDP rather than the total agency review time.
*Footnotes
The FDA’s goal is to provide comments on draft “core launch” promotional materials within 45 days of the voluntary submission of these materials by sponsors. However, some recent draft core launch promotional materials submitted for voluntary review have raised complex issues that required additional review, resulting in review times that exceeded the 45 day goal. While review timeframes for certain submissions may require more than 45 days in the immediate future, the FDA is working hard to review the majority of submissions within 45 days and to achieve overall completion percentages that are similar to those observed in the past. Sponsors are encouraged to contact the FDA with any questions and to temporarily build in additional review time for their materials.
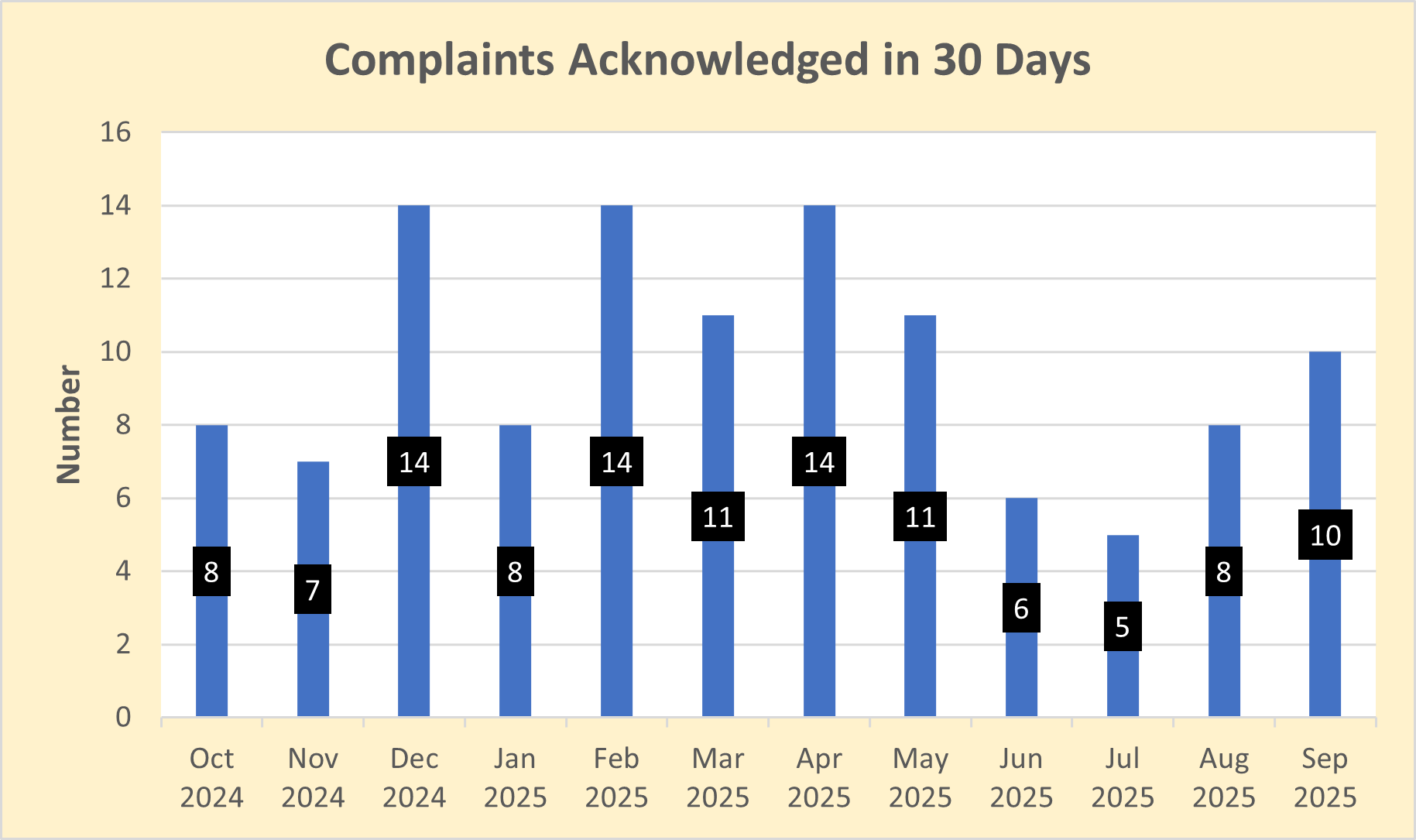
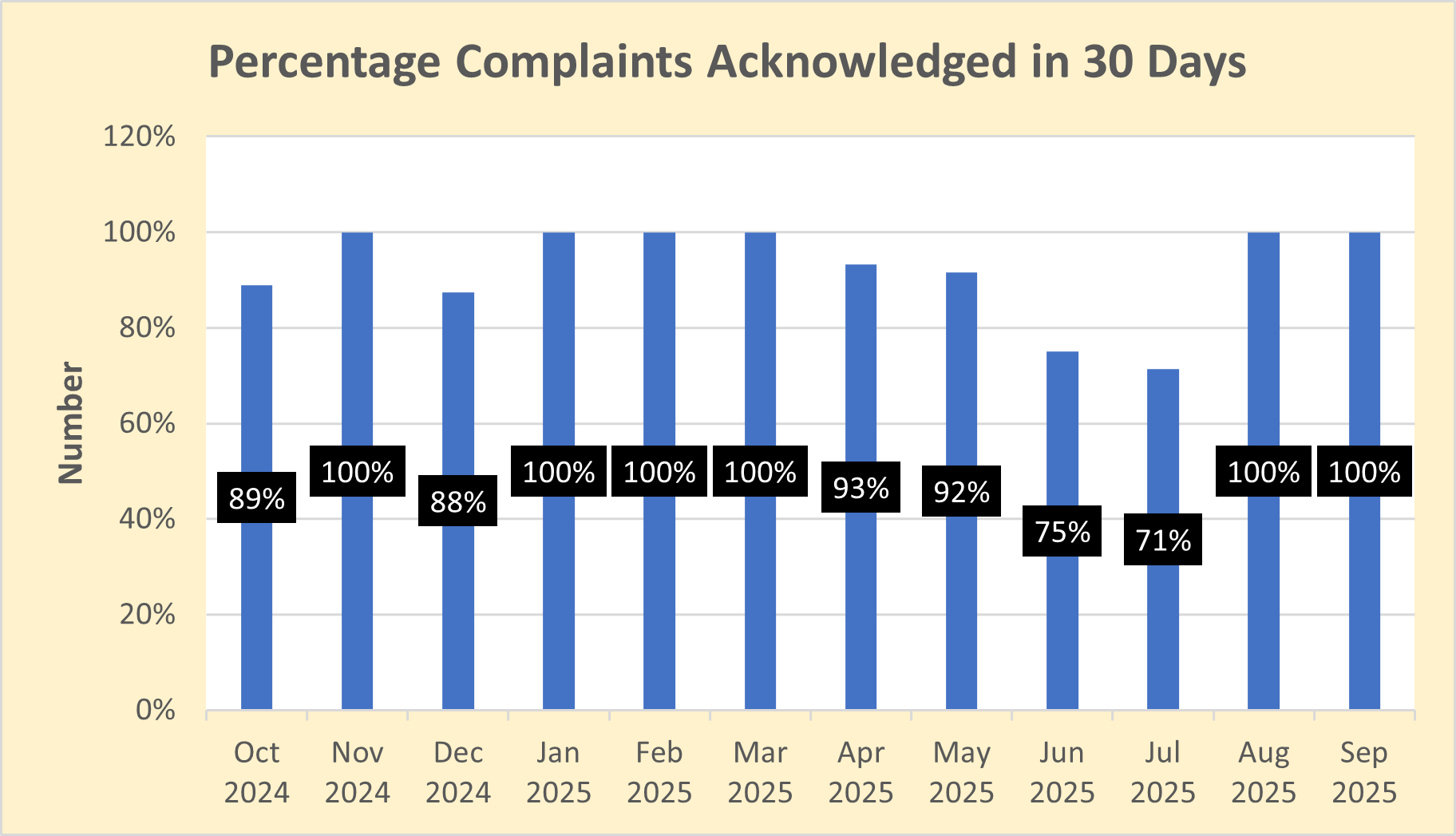
Percentage of complaints preliminarily reviewed and acknowledged within 30 days of receipt
Dictionary: OPDP receives complaints regarding potentially false or misleading promotion from health care professionals, consumers, drug sponsors, and law firms. OPDP's goal is to acknowledge receipt of these complaints within 30 calendar days.
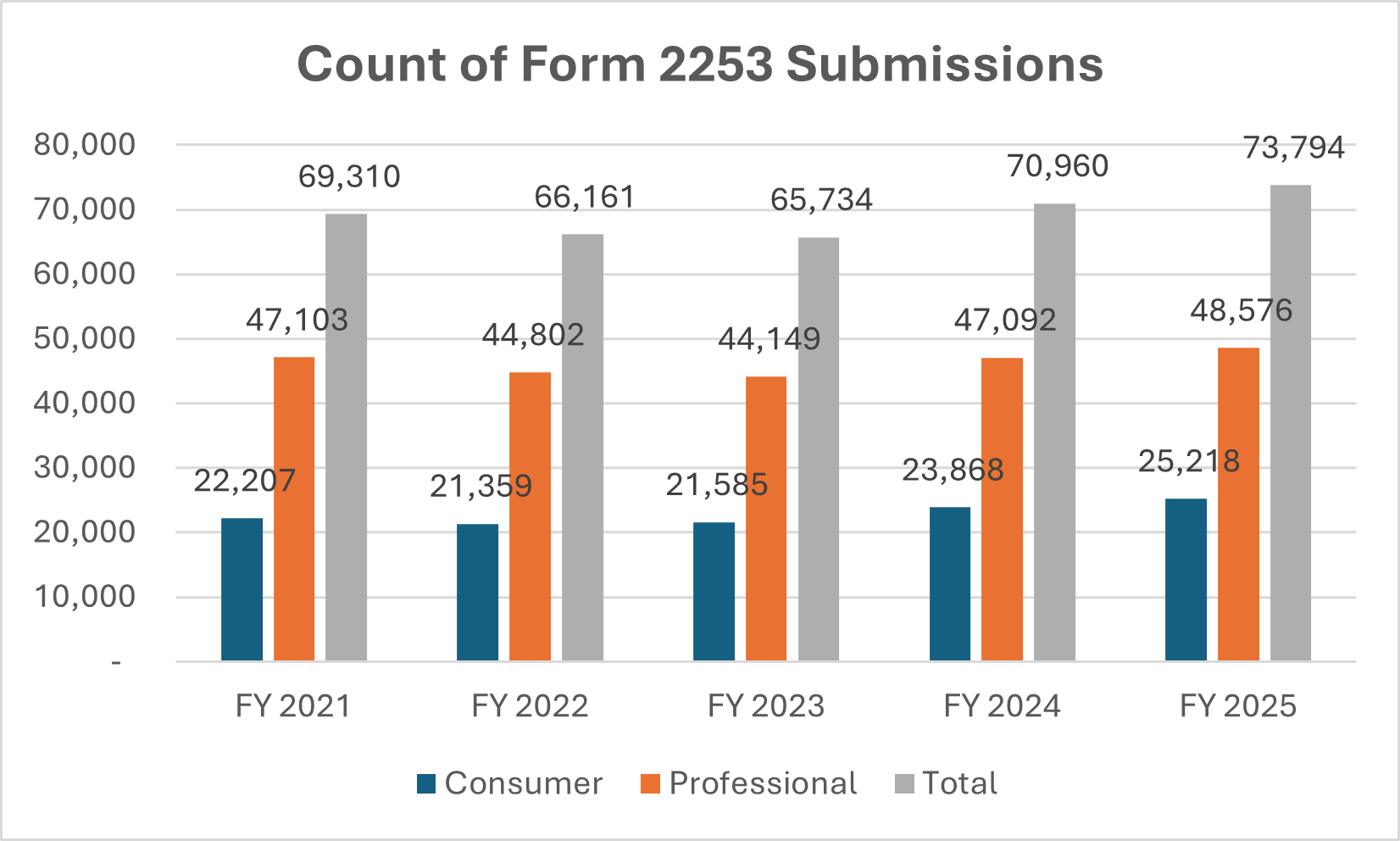
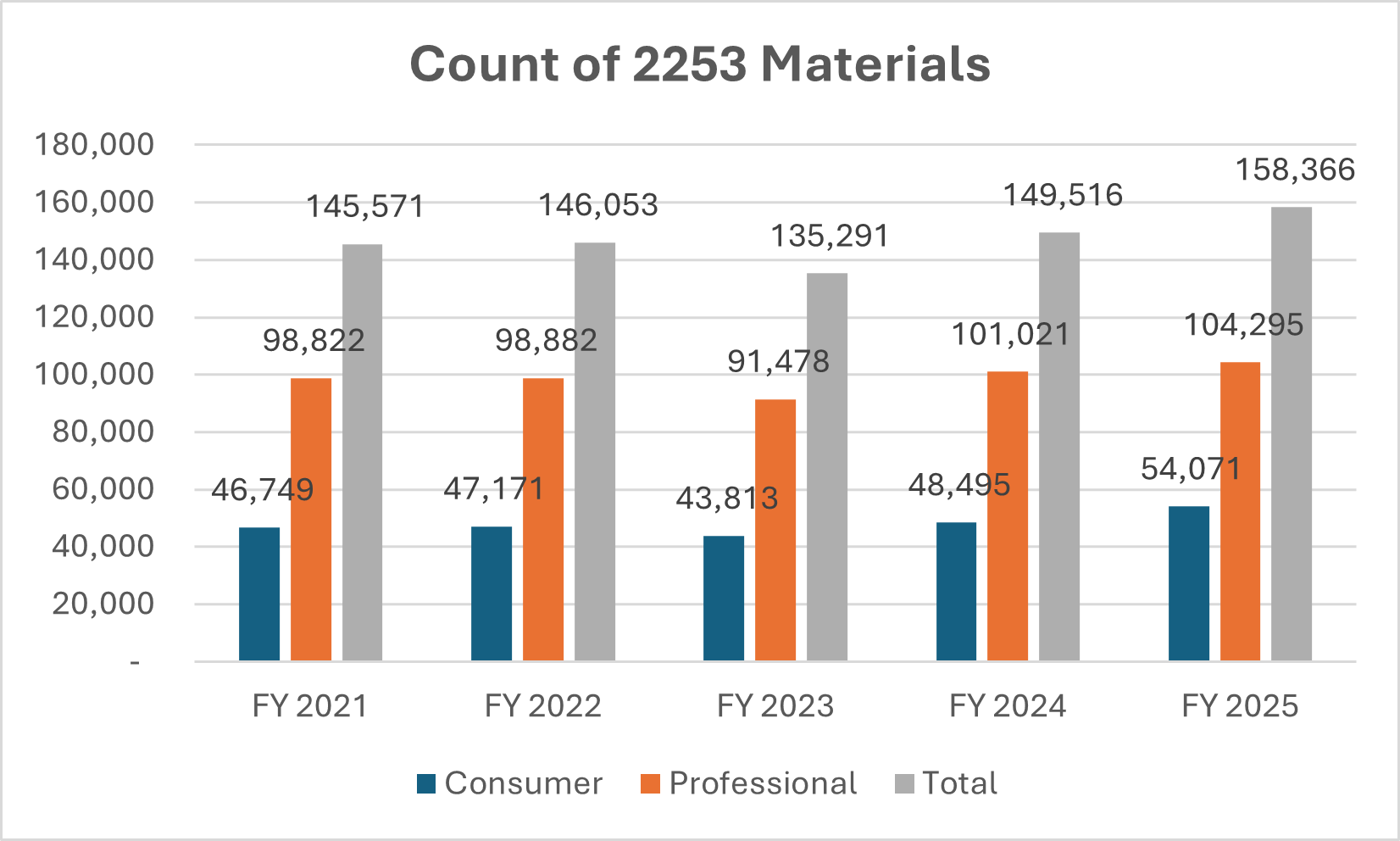
Number of Form 2253 Submissions and 2253 Materials
Dictionary: Sponsors are required to submit promotional materials under cover of Form 2253 prior to dissemination. This metric is a distinct count of Form 2253 submissions and materials included within those submissions.