COMPANY ANNOUNCEMENT
Hellolife, Inc. Issues Voluntary Worldwide Recall of Neuroveen, Respitrol, Thyroveev and Compulsin Due to Possible Microbial Contamination
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionDue to Possible Microbial Contamination
- Company Name:
- HelloLife, Inc.
- Brand Name:
-
Brand Name(s)Neuroveen, Respitrol, Thyroveev, Compulsin
- Product Description:
-
Product DescriptionHomeopathic Medicines
Company Announcement
HelloLife, Inc. in Grand Rapids, MI is initiating a voluntary recall of four different products, Neuroveen, Respitrol, Thyroveev and Compulsin, within expiry, to the retail and consumer level due to possible microbial contamination. Neuroveen has been tested and found to be contaminated with Staphylococcus saprophyticus and Burkholderia cepacia. Compulsin has been identified as containing Burkholderia cepacia.
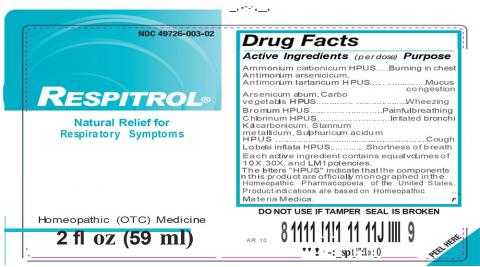
Respitrol and Thyroveev are still pending bacterial identification. Each product being recalled is for a single lot that was packaged into 2-ounce amber bottles (see table below) that were manufactured at the King Bio, Inc facility in Asheville, NC.
Administration or use of drug products with microbial contamination could potentially result in increased infections that may require medical intervention, and could result in infections that could be life threatening to certain individuals. To date, HelloLife, Inc. has not received any reports of adverse events related to the recalled products.
Specific information is as follows, all products are packaged into 2 ounce amber bottles:
|
PRODUCT |
INDICATION |
NDC |
UPC |
LOT |
EXP. DATE |
|---|---|---|---|---|---|
| Neuroveen | Natural temporary relief for nerve pain symptoms | 49726- 015-02 |
891129002804 | NV/030717D | 07/19 |
| Respitrol | Natural temporary relief for respiratory symptoms | 49726- 003-02 |
891129002729 | RE/030717E | 07/19 |
| Thyroveev | Natural temporary relief for sluggish thyroid symptoms | 49726- 025-02 |
349726000063 | TVV/030717F | 07/19 |
| Compulsin | Nervous, repetitive thought/behavior relief | 49726- 034-02 |
891129002194 | CO/030717B | 07/19 |
These products can be identified by the main label on the bottle and the lot number that is printed on the label. Product was distributed worldwide via wholesale, retail and online sales.
HelloLife, Inc. is notifying its distributors and customers by email, phone calls and written letters sent via the postal service and is arranging for replacement products where available and/or refunds. Consumers, retailers and wholesalers that have product, which is being recalled, should stop use immediately and remove all inventory from sales and contact HelloLife, Inc. to arrange for disposal and product replacement or refunds.
Consumers with questions regarding this recall can contact HelloLife, Inc. by calling 1-616-803- 7243 Monday-Friday 9:00 a.m. to 5:00 p.m. EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- HelloLife, Inc.
- 1-616-803- 7243