COMPANY ANNOUNCEMENT
PharMEDium Services Issues Voluntary Nationwide Recall of Specific Lots of Potassium Phosphate and Succinylcholine Chloride Due to a Lack of Sterility Assurance
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read AnnouncementSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionDue to Lack of Sterility Assurance
- Company Name:
- PharMEDium Services
- Brand Name:
-
Brand Name(s)PharMEDium
- Product Description:
-
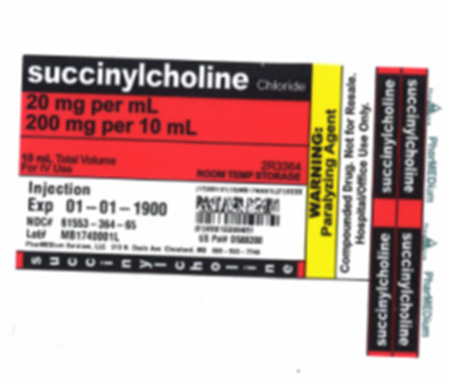
Product DescriptionPotassium Phosphate and Succinylcholine Chloride Intravia Bags
Company Announcement
PharMEDium Services is conducting a limited, voluntary recall due to Hospira Inc.’s (“Hospira”) June 15, 2017 recall announcement that microbial growth was detected during a routine simulation of the manufacturing process and therefore there was a lack of sterility assurance. The products being recalled by PharMEDium Services were compounded using certain Hospira products. The recalled products are specific lots of Potassium Phosphate and Succinylcholine Chloride. This is a secondary recall based on a Hospira's recent recall: https://www.fda.gov/Safety/Recalls/ucm563383.htm.
Per Hospira, in the event that impacted product is administered to a patient, there is a reasonable probability that the patient may experience adverse events ranging from fever, chills and malaise, to severe adverse events including systemic invasive mycoses or systemic bacterial sepsis. The possibility of a breach in sterility assurance in distributed product, while remote, cannot be eliminated.
To date, PharMEDium Services has not received any reports of product complaints and/or adverse events related to the products.
These products were not distributed directly to patients or consumers, but rather to healthcare facilities (e.g. hospitals) in the United States.
The recalled lots are as follows:
| Product |
Strength |
Lot Number |
Expiration Date |
NDC Number |
|---|---|---|---|---|
| 2K5281 | 10 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 250 mL in 250 mL Intravia Bag | 171280011S | 8/7/2017 | |
| 2K5282 | 15 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 250 mL in 250 mL Intravia Bag | 171530101S 171230071D 171170014S 171210127S 171240191S 171320190S 171570058D 171240002D 171240004D 171280041D 171290077D 171300076D 171310058D 171320059D 171360062D 171360068D 171380076D 171390064D 171390065D 171500064D |
9/3/2017 8/2/2017 7/27/2017 7/31/2017 8/3/2017 8/13/2017 9/5/2017 8/3/2017 8/3/2017 8/7/2017 8/8/2017 8/9/2017 8/10/2017 8/13/2017 8/15/2017 8/15/2017 8/17/2017 8/20/2017 8/20/2017 8/29/2017 |
61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 61553-282-11 |
| 2K5283 | 30 mMol Potassium Phosphate (Preservative Free) in 5% Dextrose 500 mL in 500 mL Intravia Bag | 171350099S | 8/14/2017 | 61553-283-03 |
| 2K5284 | 7 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 100 mL in 150 mL Intravia Bag | 171220061D 171430008D 171450015D 171510062D 171250023D |
8/1/2017 8/22/2017 8/24/2017 8/30/2017 8/6/2017 |
61553-284-48 61553-284-48 61553-284-48 61553-284-48 61553-284-48 |
| 2K5285 | 30 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 500 mL in 500 mL Intravia Bag | 171240007S 171280055D 171370011S 171360005S 171320008S 171380086D 171500071D 171280055D 171320003D |
8/3/2017 8/7/2017 8/16/2017 8/14/2017 8/13/2017 8/17/2017 8/29/2017 8/7/2017 8/10/2017 |
61553-285-03 61553-285-03 61553-285-03 61553-285-03 61553-285-03 61553-285-03 61553-285-03 61553-285-03 61553-285-03 |
| 2K5286 | 9 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 100 mL in 150 mL Intravia Bag | 171520062D | 8/31/2017 | 61553-286-48 |
| 2K5287 | 20 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 100 mL in 150 mL Intravia Bag | 171320001D | 8/10/2017 | 61553-287-48 |
| 2K5288 | 10 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 100 mL in 150 mL Intravia Bag | 171290006S 171280125S 171280006S 171360002S 171290028D |
8/7/2017 8/7/2017 8/6/2017 8/15/2017 8/8/2017 |
61553-288-48 61553-288-48 61553-288-48 61553-288-48 61553-288-48 |
| 2K5290 | 30 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 250 mL in 250 mL Intravia Bag | 171180027S 171230013S 171360008S 171560025D 171360019D 171220058D 171380085D 171430069D |
7/29/2017 8/2/2017 8/14/2017 9/4/2017 8/15/2017 8/1/2017 8/17/2017 8/22/2017 |
61553-290-11 61553-290-11 61553-290-11 61553-290-11 61553-290-11 61553-290-11 61553-290-11 61553-290-11 |
| 2K5291 | 15 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 250 mL in 250 mL Intravia Bag with Additive Cap | 171210005S 171500072D 171230008D 171560028D |
7/30/2017 8/29/2017 8/2/2017 9/4/2017 |
61553-291-11 61553-291-11 61553-291-11 61553-291-11 |
| 2K5292 | 15 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 150 mL in 150 mL Intravia Bag | 171370005S 171450012D 171510059D 171440016D 171280054D 171560027D 171320002D |
8/15/2017 8/24/2017 8/30/2017 8/23/2017 8/7/2017 9/4/2017 8/10/2017 |
61553-292-01 61553-292-01 61553-292-01 61553-292-01 61553-292-01 61553-292-01 61553-292-01 |
| 2K5295 | 15 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 100 mL in 150 mL Intravia Bag | 171320006S 171320014D 171360020D 171520063D |
8/13/2017 8/13/2017 8/15/2017 8/31/2017 |
61553-295-48 61553-295-48 61553-295-48 61553-295-48 |
| 2K5298 | 7.5 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 100 mL in 150 mL Intravia Bag | 171320109S 171210102S 171560012S |
8/13/2017 7/31/2017 9/4/2017 |
61553-298-48 61553-298-48 61553-298-48 |
| 2K5299 | 7.5 mMol Potassium Phosphate (Preservative Free) in 5% Dextrose 100 mL in 150 mL Intravia Bag | 171450017D | 8/24/2017 | 61553-299-48 |
| 2K5300 | 15 mMo1 Potassium Phosphate (Preservative Free) in 5% Dextrose | 171420030D | 8/21/2017 | 61553-300-11 |
| 2K5301 | 40 mMol Potassium Phosphate (Preservative Free) in 0.9% Sodium Chloride 250 mL in 250 mL Intravia Bag | 171220007S 171280129S 171320112S 171590010S |
7/31/2017 8/7/2017 8/13/2017 9/7/2017 |
61553-364-65 61553-364-65 61553-364-65 61553-364-65 |
| 2K5310 | 9 mMol Potassium Phosphate (Preservative Free) in 5% Dextrose 50 mL in 50 mL Intravia Bag | 171560007S 171160002S |
9/4/2017 7/26/2017 |
61553-310-41 61553-310-41 |
| 3364NO | 20 mg/mL Succinylcholine Chloride Injection (Preserved) 10 mL in 10 mL BD Syringe | 171450001D 171450002D 171450056D 171440058D 171390027D 171390026D 171430062D 171420074D 171370064D |
8/23/2017 8/23/2017 8/24/2017 8/23/2017 8/20/2017 8/20/2017 8/22/2017 8/21/2017 8/16/2017 |
61553-364-65 61553-364-65 61553-364-65 61553-364-65 61553-364-65 61553-364-65 61553-364-65 61553-364-65 61553-364-65 |
PharMEDium Services is committed to product quality and patient safety in all instances and is therefore issuing this recall.
PharMEDium Services is notifying customers of the voluntary recall by phone. Customers that have any of the affected medications that are being recalled should immediately quarantine the product, discontinue use and destroy per their hospital protocol. Customers with any of the affected medications can also reference PharMEDium Services website for more information on the specific lot numbers affected and contact information: www.pharmedium.com.
Patients and healthcare providers with questions regarding this recall can contact PharMEDium Services Clinical Pharmacist at (847) 457-2220, Monday through Friday, between 8am and 5pm Central Standard Time or via e-mail at shasan@pharmedium.com.
Patients should contact their physician or healthcare provider if they have experienced any problems that may be related to the use of these products.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.