Questions and Answers about Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL)
Q1. What does the FDA know about Breast Implant Associated Lymphoma?
A1. The FDA first identified a possible association between breast implants and the development of ALCL in 2011. At that time, the FDA knew of so few cases of this disease that it was not possible to determine what factors increased the risk. In a report summarizing the Agency's findings, we emphasized the need to gather additional information to better characterize ALCL in individuals with breast implants. In 2016, the World Health Organization designated breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) as a T-cell lymphoma that can develop following breast implants and noted that the exact number of cases remained difficult to determine due to significant limitations in world-wide reporting and lack of global breast implant sales data.
Since that time, the FDA has undertaken several steps to better understand this issue, including an in-depth review of post-approval study data, medical device reports, scientific literature and breast implant-specific registries, and public discussions. We have regularly communicated about the risks associated with breast implants and heard from patients who are concerned about their implants being connected to various health conditions. In March 2019, we discussed many important breast implants concerns in a public advisory committee meeting.
Q2. What is BIA-ALCL? Is BIA-ALCL breast cancer?
A2. Breast Implant Associated Lymphoma (BIA-ALCL) is not breast cancer - it is a type of non-Hodgkin's lymphoma (cancer of the immune system). In most cases, BIA-ALCL is found in the scar tissue and fluid near the implant, but in some cases, it can spread throughout the body. An individual's risk of developing BIA-ALCL is considered to be low; however, this cancer is serious and can lead to death, especially if not treated promptly. In most patients, it is treated successfully with surgery to remove the implant and surrounding scar tissue, and in some patients, also treatment with chemotherapy and radiation therapy.
Q3. What are the symptoms of BIA-ALCL?
A3. The main symptoms of BIA-ALCL are persistent swelling, presence of a mass or pain in the area of the breast implant. These symptoms may occur well after the surgical incision has healed, often years after implant placement.
Upon evaluation by a health care provider, evidence of fluid collection around the breast implant (seroma) is often observed. Some patient reports indicated that a lump under the skin or capsular contracture (thick and noticeable scar capsule around the implant) were present.
Q4. Where in the breast has BIA-ALCL been found?
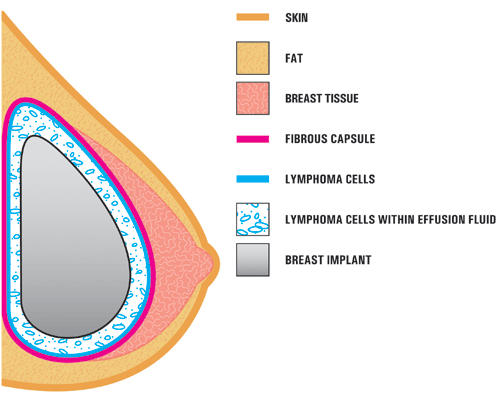
A4. In the case studies reported in the literature, BIA-ALCL is usually found near the breast implant, contained within the fibrous scar capsule, and not in the breast tissue itself. The illustration below shows the location of the ALCL in these reports. In most cases, the ALCL cells were found in the fluid surrounding the implant (seroma) or contained within the fibrous scar capsule. [Modified from Thompson et al, 2010]
Q5. Does the surface texture of the breast implant shell–smooth versus textured–increase a woman's risk of developing BIA-ALCL?
A5. We have evaluated the growing body of evidence, including new medical device reports from the U.S. and around the world on the overall number of BIA-ALCL cases. These include additional deaths only recently reported to the FDA and in the scientific literature. As a result, we have determined that:
- All patients who have breast implants or are thinking about getting them should be aware of the risk of BIA-ALCL.
- The risk of BIA-ALCL is higher for textured surface implants versus smooth surface implants.
- Certain other textured breast products, specifically certain textured tissue expanders, should not be used, and we have issued new recommendations for patients who have or have had these products.
Q6. Could certain textured tissue expanders increase the risk of BIA-ALCL?
A6. The FDA believes tissue expanders with a certain textured surface may be of concern. These tissue expanders should not be used and we have issued new recommendations for patients who have or have had these products. Tissue expanders are indicated to be used for only 6 months, and to date, there is limited information on whether temporary exposure may be associated with the risk of BIA-ALCL. Other tissue expanders that do not use the textured surface of concern are readily available in the U.S. A tissue expander is used stretch skin and other tissues before breast reconstruction after mastectomy, correction of an underdeveloped breast, scar revision, and tissue defect procedures. It is a temporary implant, placed under the breast skin or muscles of the chest to stretch skin and other tissues, and is intended to be replaced with a breast implant at a later time.
Q7. Does the fill of the breast implant–silicone versus saline–increase an individual's risk of developing BIA-ALCL?
A7. Based on the currently available data, the type of implant fill does not appear to be a risk factor for BIA-ALCL, but this has not been evaluated in a large, well-designed, epidemiologic study. To date, there has not been sufficient data to determine whether ALCL may be found more or less frequently in individuals with silicone-filled breast implants compared to individuals with saline-filled breast implants.
Q8. What should health care professionals and patients do?
A8.The FDA is recommending that health care providers continue to provide their patients routine care and support.
Health Care Professionals:
- You should immediately stop using (implanting) the breast implants and tissue expanders listed in the July 24, 2019 FDA Safety Communication; and work with your facility to return existing inventory.
- We are not recommending the routine removal of these or other types of breast implants in patients who have no symptoms.
- You should inform your patients who have the implants and tissue expanders listed in the July 24, 2019 FDA Safety Communication about the risks of serious adverse health consequences, including the potential for the development of BIA-ALCL.
- Prior to implantation of any breast implant, provide your patients with the manufacturer's patient labeling, as well as any other educational material, and discuss the benefits and risks of the different types of implants.
- Consider the possibility of BIA-ALCL when treating a patient with late onset, peri-implant changes. In some cases, patients presented with a seroma, mass, hardening adjacent to the breast implant. If you have a patient with suspected BIA-ALCL, refer the patient's case to experts familiar with the diagnosis and treatment of BIA-ALCL.
- Collect fresh seroma fluid and representative portions of the capsule and send for pathology tests to rule out BIA-ALCL. Diagnostic evaluation should include cytological evaluation of seroma fluid or mass with Wright Giemsa stained smears and cell block immunohistochemistry/flow cytometry testing for cluster of differentiation (CD30) and Anaplastic Lymphoma Kinase (ALK) markers.
- Develop an individualized treatment plan in coordination with experts familiar with the diagnosis and treatment of BIA-ALCL. Consider current clinical practice guidelines, such as those from the Plastic Surgery Foundation or the National Comprehensive Cancer Network (NCCN) when choosing your treatment approach.
Patients:
- If you have no symptoms, we are not recommending the removal of the implants and tissue expanders listed in the July 24, 2019 FDA Safety Communication; or other types of breast implants due to concern related to the risk of developing BIA-ALCL.
- Know the symptoms of BIA-ALCL, primarily persistent swelling, presence of a mass or pain in the vicinity of the breast implant and monitor the area around your breast implants for any changes.
- If you experience any of these symptoms or other changes, talk to your healthcare provider regarding the need for further evaluation. Evaluation for BIA-ALCL typically involves a physical exam, imaging, and/or assessment of the fluid or tissue around the breast implant. It is important to undergo an evaluation to diagnose BIA-ALCL since a confirmed BIA-ALCL diagnosis may change the type of operation that should be performed.
- Patients with confirmed BIA-ALCL should undergo implant removal with removal of the surrounding scar capsule, which is a more extensive operation than implant removal alone.
- As with any implanted device, it is good to keep a record of the device manufacturer and implant model name. You may have received this information on a patient device card from your surgeon. If you would like to obtain the manufacturer name and model of your implants, consider asking your surgeon or obtaining the record of your surgery (operative notes) from the facility where it was performed.
- Understand that most cases of BIA-ALCL occur years after breast implant placement and present with symptoms or changes around the breast implant. Talk to your surgeon about your risk of developing BIA-ALCL.
- If you are considering breast implants, please see these important recommendations.
We will continue to report on significant findings as new information and analyses become available.
Q9. How can health care professionals report cases of BIA-ALCL in their patients?
A9. Health care professionals should:
- Report all cases of BIA-ALCL in individuals with breast implants to MedWatch, the FDA Safety Information and Adverse Event Reporting program.
- Health care personnel employed by facilities that are subject to FDA's user facility reporting requirements should follow the reporting procedures established by their facilities. Prompt reporting of adverse events can help the FDA identify and better understand the risks associated with medical devices. In some cases, the FDA may contact you for additional information. The FDA will keep the identities of personnel reporting the event and the patient confidential.
- Submit case reports of BIA-ALCL to the Patient Registry and Outcomes For breast Implants and anaplastic large cell Lymphoma etiology and Epidemiology (PROFILE) Registry to contribute to a better understanding of the causes and treatments of BIA-ALCL.
Q10. If an individual is considering breast implants, what should they do?
A10. There are several important things to consider before deciding to undergo breast implant surgery. This list is available from the FDA at (revised Things to Consider Before Getting Breast Implants in Risks and Complications) to help you be fully informed if you are considering breast augmentation, reconstruction with an implant, or revision (replacement) of an implant you already have. Most importantly, you and your surgeon should discuss your goals and expectations about having breast implants, the benefits and risks, the need to monitor your implant for complications for as long as you have them, and eventual removal or replacement.
Q11. What actions will the FDA continue to take?
A11. The FDA continues to collect and evaluate information about BIA-ALCL in individuals with breast implants and who have used tissue expanders.
On an ongoing basis, we:
- Receive and review medical device reports (MDRs).
- Review the current medical literature.
- Exchange information with other U.S. and international regulators and scientific experts.
- Review data from the Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma (ALCL) Etiology and Epidemiology (PROFILE Registry) (a collaborative effort with the American Society of Plastic Surgeons (ASPS) and the Plastic Surgery Foundation (PSF)).
- Review information that breast implant manufacturers include about BIA-ALCL in their patient and health care professional labeling.
- Review information provided from on-going post-market studies.
- Monitor adverse events from other real-world data (e.g. National Breast Implant Registry).
Q12. What actions have been taken by Professional Societies and Regulatory bodies outside the US?
A12. The World Health Organization recognized breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) as a unique form of ALCL that can develop following breast implant, implantation.
Professional organizations, including the Plastic Surgery Foundation and the National Comprehensive Cancer Network (NCCN) published information to help physicians understand the disease and provide diagnosis and treatment.
On February 12, 2019 Health Canada announced it will be updating its safety review of breast implants.
On April 4, 2019, the Medicines and Healthcare products Regulatory Agency announced its recommendations for patients and health care providers.
On April 4, 2019, the French National Agency for Medicines and Health Products Safety (ANSM) announced its decision to, as a precautionary measure, withdraw from "macrotextured" breast implants and breast implants with polyurethane-coated surfaces marketed in France. The ANSM does not recommend preventative explanation for women with these implants
On July 11, 2019 The Australian Therapeutic Goods Administration (TGA) reported announced it has completed assessment of textured breast implants available in Australia or exported from Australia and proposed regulatory actions.
Q13. Where can we find more information?
A13. Additional information can be found in FDA's recent communications and our breast implant webpage.