GUIDANCE DOCUMENT
Guidance for Industry: Questions and Answers About the Food Additive or Color Additive Petition Process April 2011
- Docket Number:
- FDA-2020-D-1917
- Issued by:
-
Guidance Issuing OfficeHuman Foods Program
This guidance represents the Food and Drug Administration's (FDA's) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations.
I. Introduction
This guidance provides industry with a convenient place to find answers to frequently asked questions about submitting a food additive or color additive petition. This guidance is a revision of the April 2006 guidance titled "Questions About the Petition Process." This guidance updates information and provides a response to a question regarding disclosing information in a food additive or color additive petition.
FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.
II. Questions and Answers
- When do I need to submit a food additive or color additive petition?
- Who do I contact to determine if I need to submit a petition to the Office of Food Additive Safety?
- If I determine I need to submit a petition, where do I start?
- Will the information in my petition be disclosed if requested under the Freedom of Information Act?
- After I submit a petition, who is the point of contact for my petition?
- How do I prepare the scientific documentation to support the safe use of my proposed additive?
- What are the basic elements of a safety assessment for an additive?
- What is meant by the administrative record for an additive?
- What are the essential elements of a good food and/or color additive petition?
- How do I prepare a petition that contains the appropriate chemistry information?
- How do I prepare an adequate environmental assessment?
- How do I determine the type of toxicological information necessary to support the safety of my proposed food additive?
- How do I design toxicological studies that will provide the information necessary to establish safety?
- What else is recommended to help make my submission of toxicological information successful?
- How should the results of the toxicity studies be presented and discussed?
- What are some factors to consider when assessing the significance of treatment-related effects in toxicity studies?
- Can you give a very simplified overview of the process a petition undergoes after it is received by OFAS?
- What are some characteristics of the food additive and color additive approval process?
- After a notice of filing is published, how long does it usually take until a final regulation is published in the Federal Register?
A. When do I need to submit a food additive or color additive petition?
Any food additive intended to have a technical effect in food and any color additive for use in foods, drugs, cosmetics, or medical devices that are in contact with the body for a significant period of time is deemed unsafe unless it either conforms to the terms of a regulation prescribing its use or to an exemption for investigational use. A petition for a food additive or color additive is submitted to request issuance of a regulation allowing new uses of the additive and must contain the necessary supporting data and information.
Prior to submitting a petition, you should verify that your additive is not already regulated for your intended use by consulting FDA's regulations in Title 21 of the Code of Federal Regulations (CFR), Parts 170-199 for food additives, and Parts 73 and 74 for color additives.You may also want to consult a food additive database called "Everything Added to Food in the United States (EAFUS)."If a food additive is regulated for use in food, it will be listed in EAFUS with a "regnum" (a regulation in 21 CFR).To be acceptable for a particular application, an additive must meet the provisions (e.g., identity, specifications, and use limitations) in the applicable regulation.
B. Who do I contact to determine if I need to submit a petition to the Office of Food Additive Safety?
Contact the Office of Food Additive Safety in one of the following ways:
Mail:
FDA/Center for Food Safety and Applied Nutrition
HFS-200
5001 Campus Drive
College Park, MD 20740-3835
Email: premarkt@fda.hhs.gov
Your correspondence will be assigned to a Consumer Safety Officer (CSO) who will help you determine if you need to submit a petition.
C. If I determine I need to submit a petition, where do I start?
We have a number of guidance documents available on FDA's website, including Guidance for Industry: Pre-Petition Consultations for Food Additives and Color Additives, that can assist you with getting started.
D. Will the information in my petition be disclosed if requested under the Freedom of Information Act?
Information submitted as part of a petition is subject to the disclosure provisions under 21 CFR part 20 and 21 CFR 71.15(a) or 21 CFR 171.1(h), depending on whether the petition is for a color additive or a food additive. FDA recommends that the petitioner identify or redact those portions of the petition that the petitioner considers to be trade secret or confidential commercial or financial information in one of the triplicate copies (quadruplicate, if intended uses include use in meat, meat food products, or poultry products) of the petition required in 21 CFR 71.1(a) and 21 CFR 171.1(c) to be submitted to FDA.FDA may not agree that all identified or redacted information is protected from disclosure under 21 CFR part 20.
E. After I submit a petition, who is the point of contact for my petition?
A Consumer Safety Officer (CSO) is assigned to each petition.The CSO is the point of contact for consultation with FDA technical experts.The CSO also arranges meetings and provides information about the status and progress of your submission.
F. How do I prepare the scientific documentation to support the safe use of my proposed additive?
We have a number of guidance documents available to help you prepare your petition.
- Toxicology Guidance Documents: 1993 Draft Redbook II, Redbook 2000 and Templates for Reporting Toxicology Data.
- Chemistry Guidance Documents.
- Guidance Documents for Color Additive Petitions
- Environmental Guidance Documents
A note about guidance vs. regulations:Regulations are requirements defined by law in the U.S. Code of Federal Regulations (CFR). The formats required for a food additive petition and color additive petition are outlined in 21 CFR 171.1 and 71.1, respectively, and must be followed exactly.Unlike regulations, FDA's guidance documents, including this guidance, do not establish legally enforceable responsibilities. Instead, guidances describe the Agency's current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The guidance documents listed in the response to this question provide assistance to the petitioner in preparing information for scientific review. These documents are intended to help the petitioner submit information in a consistent and appropriate manner.
G. What are the basic elements of a safety assessment for an additive?
The basic elements are:
- Identity
- Probable exposure
- Evaluation of safety
- Limitations of conditions of use (may be necessary to ensure safe use)
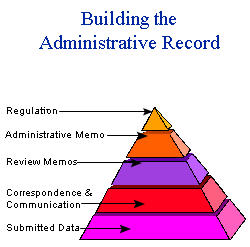
H. What is meant by the administrative record for an additive?
The administrative record includes the components outlined below.
- Data are submitted by the petitioner
- There is communication between FDA and the petitioner
- FDA reviews the data as clarified by the communication, and prepares memoranda
- FDA publishes a final regulation, which authorizes a specific use of the additive, must withstand legal challenge, and bears FDA's credibility.
I. What are the essential elements of a good food and/or color additive petition?
- The identity and composition of the additive
- Proposed use
- Use level
- Data establishing the intended effect
- Quantitative detection methods
- Estimated exposure from the proposed use (in food, drugs, cosmetics, or devices, as appropriate)
- Full reports of all safety studies
- Proposed tolerances (if needed)
- Environmental information (as required by the National Environmental Policy Act (NEPA), as revised (62 FR 40570; July 29, 1997)
- Fee (for color additive petitions only)
- Ensure that consistent information is presented throughout all sections of the petition, including those pertaining to:
- chemistry,
- toxicology,
- environmental science, and
- any other pertinent studies (e.g., microbiology)
J. How do I prepare a petition that contains the appropriate chemistry information?
- Consult the Chemistry Guidance Documents. If for a color additive, consult the Guidance for Industry: Color Additive Petitions- FDA Recommendations for Submissions of Chemical and Technological Data on Color Additives for Food, Drugs, Cosmetics or Medical Devices.
- Evaluate the appropriateness of studies conducted, and the effects significant deviations from FDA's guidance documents has on them. These deviations and effects should be discussed in the petition.
- The design of any study and assessment of the results should be based on sound scientific principles, not rote adherence to guidance.
- Significant deviations from appropriate guidance should be justified and possible effects on the study discussed.
- Consult with the Office of Food Additive Safety (OFAS) for protocol review BEFORE initiating any studies to ensure:
- that the proper test data will be submitted,
- that the analytical methodology will be adequate, and
- that the validation methodology will be adequate
- Submit all supporting raw data, including that for:
- samples,
- standards,
- construction of calibration curves, and
- determination of limits of detection (LOD) and limits of quantitation (LOQ).
- Submit a complete description of the analytical methods, including:
- sample workup,
- preparation of standards, and
- example calculations
- Submit all other relevant information, for example:
- technical brochures,
- material safety data sheets (MSDS),
- methodology and calculations for estimating intake, and
- references, as appropriate (in English)
- Discuss the results:
- Interpretations and conclusions should be scientifically sound and supported by the data.
- Data interpretations should be scientifically sound, clearly explained and supported by peer-reviewed references.
K. How do I prepare an adequate environmental submission?
The guidance listed below is intended to assist submitters in the preparation of claims of categorical exclusion and environmental assessments (EA). The guidance recommends the types of information that would be helpful to the Agency's review of environmental submissions and refers to the National Environmental Policy Act (NEPA) implementing regulations in 21 CFR part 25 that were revised in July 29, 1997.
If extraordinary circumstances indicate that the proposed action might have significant environmental impacts, the Agency will require the preparation of an EA for any normally excluded action. Examples of extraordinary circumstances are discussed in theguidance. In most instances, a categorical exclusion can be determined or confirmed by review of other information in the submission. In limited instances, CFSAN will recommend additional information to establish that the criteria for a categorical exclusion have been met, particularly for exclusions claimed under 21 CFR 25.32(i) and (q).
When testing is necessary, consult the Environmental Assessment Technical Assistance Handbook.
Please contact the Environmental Review Team at premarkt@fda.hhs.gov for assistance in preparing a claim of categorical exclusion or an EA and before doing environmental fate and effects testing. If an EA is necessary, we recommend that you:
- Completely and accurately address all format items in the specific guidance document.
- Ensure that the environmental assessment (EA) is consistent with earlier sections of the petition:
- For example, make certain that the subject additive and proposed use in the EA are the same as in sections A, C, and G of the petition.
- Support all conclusions with data, calculations, or information from the scientific literature.
- Include appropriate references (in English).
- Place any confidential information in an appendix to the petition.
- Further suggestions:
- Contact the Environmental Review Team early in the petition preparation process.
- Use a worksheet/checklist to make sure all elements of the EA are complete.
- Look at previously prepared/regulated petitions for examples of successful EAs.
L. How do I determine the type of toxicological information necessary to support the safety of my proposed food additive?
- Consult FDA's Toxicology Guidance (1993 draft Redbook and Redbook 2000).
- Additional Toxicology Guidelines may be useful:
- FDA's Good Laboratory Practice (GLP) for Non-Clinical Studies regulations in 21 CFR part 58.
- Evaluate the appropriateness of studies conducted, and the effects significant deviations from FDA guidance documents has on them. These deviations and effects should be discussed in the petition.
- The design of any study and assessment of the results should be based on sound scientific principles, not rote adherence to guidance.
- Significant deviations from appropriate guidance should be justified and possible effects on the study discussed.
M. How do I design toxicological studies that will provide the information necessary to establish safety?
We recommend that you consult with OFAS. Contact OFAS as outlined in Question II.B. of this guidance and a Consumer Safety Officer (CSO) will be assigned to your submission as the point of contact.Toxicology reviewers will be assigned:
- to determine what toxicology information is needed to support safety,
- for complete or partial protocol review (for example, is the test substance appropriate?), and
- to discuss appropriate ways of dealing with significant protocol deviations during toxicity studies.
N. What else is recommended to help make my submission of toxicological information successful?
- Provide an inventory of studies and an overview of existing toxicological data.
- Submit the results of a toxicological literature search on the additive and major impurities along with the search parameters that were used. We recommend databases such as Science Direct, Pub Med, and Tox line.
- Submit full reports of all available unpublished toxicity studies on the petitioned substance as well as published toxicity studies pivotal to the safety assessment.
- Uniquely identify each toxicity study.
- Provide English language copies of studies or literature cited.
O. How should the results of the toxicity studies be presented and discussed?
- Include data that will allow the reviewer to independently evaluate and verify the results claimed. For example:
- the raw data on the concentration and stability of the test substance as administered,
- the individual animal data for all endpoints, and
- the pathologist's narrative and summary tables of non-neoplastic and neoplastic lesions.
- Discuss results. For example:
- comment on statistically significant effects,
- provide historical/control data as appropriate, and
- DO NOT make unsupported statements. Data interpretations should be scientifically sound, clearly explained and supported by peer-reviewed references.
P. What are some factors to consider when assessing the significance of treatment-related effects in toxicity studies?
When assessing treatment-related effects, these are some of the factors to consider when determining the significance of differences between treated and control groups:
- dose-related trends,
- reproducibility,
- related findings,
- the magnitude and types of differences, and
- occurrence in both sexes.
From: Principles and Methods of Toxicology, Third Edition (1994), Chapter 17, page 668.
Q. Can you give a very simplified overview of the process a petition undergoes after it is received by OFAS?
The schematic below represents an extremely simplified picture of some of the elements that contribute to the food additive petition review process. This is an iterative process in which some steps may be repeated several times until there is an Agency consensus that there is a reasonable certainty of no harm from the petitioned use of the additive.
A new petition is received
↓
- OFAS evaluates the petition for adequacy of filing (if adequate, a filing notice is published in the Federal Register)
- The need for review outside CFSAN is determined
↓
The Consumer Safety Officer distributes appropriate parts of the petition to experts for evaluation:
↓
| Chemistry Review | Toxicology Review | Environ- mental Review | Evaluation by Other Govern- ment Experts | Evaluation by "Outside" (non-government) Experts |
|---|---|---|---|---|
| The identity, specifications, manufacturing, use, functionality, and exposure estimate are verified. Analytical methods are evaluated. | Safety studies are evaluated | Environmental data are evaluated. | As needed on a case-by-case basis | As needed on a case-by-case basis |
| Probable exposure is calculated. | Acceptable exposure level is determined. | An environmental memo is prepared. | A report is prepared. | A report is prepared. |
↓
Decisions are made:
- a tentative safety conclusion is reached,
- advisory committee input is received, if necessary,
- the final safety evaluation is made,
- the administrative record is compiled, and
- a Federal Register final rule is drafted
↓
The final Agency review is completed.
The final regulation is published in the Federal Register.
The regulation appears in the Code of Federal Regulations.
R. What are some characteristics of the food additive and color additive approval process?
- Regulations issued are "generic" and are not "licenses" in the drug/device sense:
- anyone in compliance with the conditions of use laid out in the regulation may use the additives for the regulated use
- therefore, careful consideration of these conditions of safe use is required prior to any decision.
- Approvals are safety-based only:
- there is no explicit balancing of risks vs. benefits, and
- safety, per se, is based on the statutory standard.
- The statute requires that a food additive or color additive cannot be used until a regulation is in effect that allows the use, as announced in the Federal Register. For food additives the regulation is effective when the final rule is published. For color additives the final rule becomes effective 31 days after the final rule is published.
- formal rulemaking requires an opportunity for objections, hearings, and court challenge, and
- a detailed Federal Register preamble must be prepared laying out the rationale of the action.
S. After a notice of filing is published, how long does it usually take until a final regulation is published in the Federal Register?
The average time between submission until a final rule is published for a direct food additive petition is 24 months and for color additive petitions, the approval process varies significantly.The time to final rule for a petitioned chemical is dependent on:
- the size (number of pages to examine) of the petition,
- the complexity of the petition,
- the number of supplements (subsequent submissions), and
- the number of results to examine
Related Information
Submit Comments
You can submit online or written comments on any guidance at any time (see 21 CFR 10.115(g)(5))
If unable to submit comments online, please mail written comments to:
Dockets Management
Food and Drug Administration
5630 Fishers Lane, Rm 1061
Rockville, MD 20852
All written comments should be identified with this document's docket number: FDA-2020-D-1917.