MPM: V-7. Seafood
Macroanalytical Procedures Manual (MPM) Main Page
Contents
- Method for Microscopic Detection Of Fish Tissue In Crab Meat or Crab Cakes
- Method for Determination of Parasites in Fin Fish
A. Method for Microscopic Detection Of Fish Tissue In Crab Meat or Crab Cakes (V-27)
(1) Scope
This method describes a microscopic procedure for the detection of fish tissues which may be substituted in whole or in part for crab meat. Crab meat products are prepared from the meat derived from any of several species of edible crabs, including blue, king, queen, tanner, Dungeness, red, and stone crabs, which are members of the Class Crustacea in the Phylum Arthropoda.
(2) Applicable Documents
(3) Defect
Some manufacturers of crab cakes may add fish meat to their product. With the following method, it is possible to detect the presence of as little as 1% fish meat in experimental batches.
(4) Procedure: Microscopic Determination of Fish Tissue Added to Crab Products
- Sample Preparation and Visual Examination -- Weigh subsample and place the material in a shallow dish or pan. Spread out and examine with the naked eye. The muscle fibers of cooked crab are bluish white and have a translucent appearance. Boiled fish meat has a dead or chalky white appearance. Pick out any chalky white lumps of meat for microscopic study as well as some of the non-chalky white material.
- Microscopic Examination -- Mount bits of the muscle fiber in acidified chloral hydrate-glycerol solution on slides, warm slide to clear tissues, and examine with a compound microscope. The striations on the fish muscle are indistinct as contrasted with the distinct striations of crab muscle fiber. Weigh any foreign tissues found and estimate percent present in the product.
- Report -- Report presence of any fish meat and the approximate percent (by weight) found.
References
(1) Food Microscopy, J. G. Vaughn, Ed., Chapter 9, "Fish," Academic Press, New York, 1979.
(2) Freeman, C. C., "Cod or Crab," FDA Papers, Sept. 1967, pp 20-22.
B. Method for Determination Of Parasites in Fin Fish (V-28)
(1) Scope
This method describes procedures for the determination of parasites in fish by visual examination. Gross visual examination is effective when the parasites are visible on the exposed surfaces of the fish or when the fish flesh is sufficiently transparent for the parasite to be seen against the background of food material. Up to 95% of the parasitic nematodes recovered by methods for digestion of fish can be detected by macroscopic examination. Macroscopic examination for parasites may be performed in conjunction with organoleptic examinations for decomposition.
(2) Applicable Documents
(3) Defects
Parasites in the edible flesh of fish are a naturally occurring defect. Among the parasites that infest fin fish are species of the Protozoa, three phyla of helminths and the parasitic copepods of the Class Crustacea.
- Protozoa -- Although protozoa are usually microscopic in size, certain aggregated protozoans can occasionally be detected through the gross visual examination of fish. Sporozoan cysts (Wardia spp. in fresh water; Glugea spp. in brackish and ocean water) present in fish viscera or muscles are examples. They are noticeable because of the size of the cyst and because the cysts are opalescent and sometimes pigmented.
-
Helminths -- The three distinct phyla of helminths found as parasites in fin fish are the Platyhelminthes, Nematohelminthes, and Acanthocephala.
- (i) Flatworms (Platyhelminthes). This phylum includes monogeneans which usually attach to the gills, scales, or fins of fin fish and trematodes (flukes) which form disk-shapedcysts near the skin of thefish. Trout and salmon are frequently parasitized by Discocotyle salmonis (Monogenea). Larval spaghetti worms [Poecilancistrium robustum (Cestoda) occur as large cysts in the flesh of drum and other fish of the Gulf and Atlantic coasts of the United States.
- (ii) Roundworms (Nematoda). Pollock and other coastal fish of Norway may be heavily parasitized by larvae of Hysterothylacium aduncum. Cod are routinely candled in several countries for detection and removal of macroscopic nematodes before packaging.
- (iii) Spiny-headed worms (Acanthocephala). These worms live in the intestine and are attached to the wall by a protrusible proboscis covered with recurved hooks; the worms vary in length from less than an inch to more than a foot. The body of individuals from most species is elongate, flattened and capable of extension. No digestive tract is present at any stage of their life cycle; food is absorbed directly from the host's intestine.
- Copepoda -- Copepods are free-swimming microcrustacea. They are the most numerous marine crustaceans in many habitats, both in species and as individuals. Copepods are usually bottle-shaped and generally range in size from less than 1 mm up to 50 mm; One genus, Pennela, reaches 250 mm in length. Many species are fish parasites. Among members of the Order Lernacopodorda, the females at one stage of development become immovable in the tissues of the host fish.
(4) Procedure: Determination of Parasites in Processed Fin Fish
-
Sample Preparation -- Each subsample should consist of 10 randomly selected 200 g portions of fish flesh per lot (portions may require compositing of fish weighing less than 200 g each). Breaded fish portions should be treated as in (iii) below to remove the breading and obtain the ten 200 g portions of fish flesh. Subsamples should be analyzed according to the multiple sampling plan [see (4)e. below]; following this sampling plan, analysis of up to three subsamples from each lot may be required. Prepare subsamples as described below:
- (i) Fresh White-Fleshed Fish -- Remove fish skin and cut into fillets 20 mm thick or less.
- (ii) Fresh Fish with Pigmented Flesh or Processed or Frozen Fish -- Do not fillet. Prepare breaded products as in (iii) below.
- (iii) Removal of Breading -- Frozen products should be thawed at room temperature in a beaker of appropriate size. After thawing, pour a hot (50oC) solution of 2 % sodium lauryl sulfate in water over the fish in increments of 100 mL per 300 g of product. Stir with a glass rod for 1 min. Allow to stand for at least 10 min or until breading separates from the flesh. Transfer individual portions to a No. 10 sieve nested over a No. 40 sieve. Wash the breading through the No. 10 sieve with a gentle stream of warm tap water. Examine the No. 40 sieve containing the breading periodically, using UV light [see caution, part (4)c. below]. Parasites will appear fluorescent under this light. Note any parasites detected and record for the report. Discard the breading by backflushing the No. 40 sieve with tap water.
- Candling of White-Fleshed Fish -- Examine both sides of each prepared fillet on a light table. The intensity of the light must be sufficient to be transmitted through the flesh. Parasites should appear as irregularly spaced dark shadows in the translucent flesh. Parasites may be isolated for identification by dissection of the fish flesh. Isolated parasites should be fixed by the methods outlined in the specific parasite descriptions [see (4)d. below]. Suspect specimens which are not identified should be fixed in 10% formalin as in (4)d.(i) below.
- Ultraviolet Examination of Dark-Fleshed Fish -- Visually examine each portion, de-breaded or de-skinned as necessary, on both sides under a desk lamp or similar light source. A magnifying desk lamp (II.(7)) may be used. Report findings as described below. Conduct UV examination in a darkened room. Examine each portion on both sides with reflected longwave UV light (366 nm wavelength). Parasites should fluoresce blue or green under this wavelength light. Fish bones and connective tissues, which also fluoresce blue, may be differentiated by their regular distribution and shape. Bone fragments will be rigid when probed. For UV examination of breading, see 7.B.(6)a.(v) above. Caution: Never expose unprotected eyes to UV light from any source, either direct or reflected. Always wear appropriate eye protection, such as goggles having uranium oxide lenses, welder's goggles, etc., when such radiations are present and unshielded. Keep skin exposure to UV radiations to a minimum.
-
Fixation of Parasites -- Parasites from lots which are actionable shold be fixed as described below and submitted to FDA headquarters for identification.
-
(i). Protozoa -- Species of the microsporidian genera Glugea, Plistophora, and Nosema may be encountered as encapsulations in the fish flesh. The parasite-containing capsules are usually white and more or less globular, ranging in diameter from less than 1 mm to 5 mm. Suspected protozoan cysts should be fixed in 10% buffered formalin [10 parts 37-40% formaldehyde, 90 parts 0.1 M phosphate buffer (pH 6.8-7.2)] for further identification.
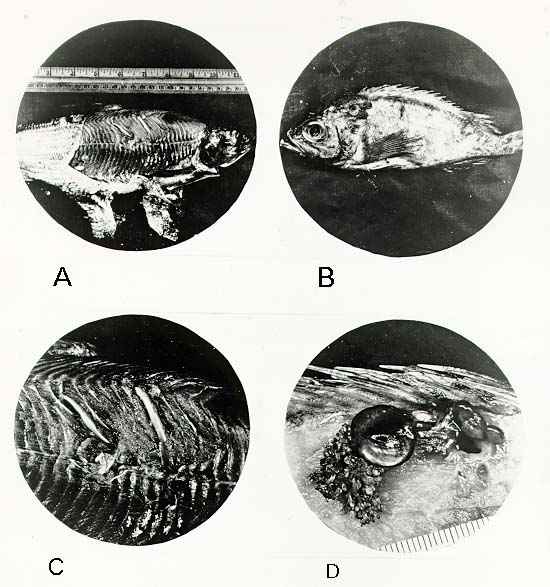
CYSTS CONTAINING PARASITES IN FIN FISH
A -- Cysts containing tapeworm larvae (Triaenophorus on tullibee(0.3X))
B -- Female copepod (Sphyrion lumpi on rosefish (0.3X))
C -- Enlarge view of A (1X)
D -- Enlarged view of B showing internal attachment by means of the Sphyrion (1.5X))
- (ii). Trematodes (Flukes) -- Larvae of trematodes (metacercaria) are frequently found at or near the skin of the fish. The disk-shaped cysts of these flatworms vary in diameter from 1 mm to 3 mm and frequently are darkly pigmented (brown or black). The lanceolate larvae usually have two suckers, one anterior and the other midventral. Trematodes should be fixed in a mixture of formalin, alcohol, and acetic acid (FAA) for further identification. (FAA consists of 10 parts 37-40% formaldehyde, 70 parts 95% ethanol, 15 parts water, and 5 parts acetic acid.)
- (iii). Cestodes -- The elongate, flattened larvae (pleurocercoids or spargana) are white to cream-colored and have an anterior holdfast organ. Unencapsulated pleurocercoids of Diphyllobothrium latum L., the broad fish tapeworm of man, are 1 to 5 mm in width and up to 20 to 40 mm in length. The encapsulated pleurocercoids of Triaenophorus crassus Rudolphi are 2 to 4 mm wide and may be fixed in FAA for identification.
- (iv). Nematodes -- Nematode larvae are cylindrical and highly variable in size, ranging from less than 0.25 mm to more than 100 mm in length and from 0.01 to 2 mm in diameter. Different species have different amounts of pigmentation; some appear white or cream-colored, others pinkish to red, and some tan or brownish. Some types are encapsulated and others are not; the same kind of nematode may even have some individuals encapsulated and others free in the same host. Nematodes are frequently coiled in the flesh of the fish, either in elongated spirals like a corkscrew or in flat coils. For identification, isolated nematodes should be fixed in glacial acetic acid for at least 1 hr. They should be transferred to 70% ethanol with 10% glycerol for storage and/or shipment.
- (v). Acanthocephala -- For further identification, each larva must be dissected from its capsule and placed in distilled water for 1 hr at 2-5o C. This procedure relaxes the worm; the hydrostatic pressure causes the proboscis to evert. Cystacanths with everted proboscis should be fixed in warm (50oC) FAA.
-
(vi). Copepods -- These crustaceans are seldom found complete on marketed fish; however, the mouthparts may be found in ulcerous lesions 20 to 30 mm in diameter at the surface of the fish flesh. For identification, the affected area is cut from the flesh and fixed in 95% ethanol.
Editor's Note: Agency policy concerning sampling and criteria for regulatory action has changed considerably since the original publication of the following Multiple Sampling Plan. The plan may no longer be valid in many cases. Readers are advised to consult the Office of Seafood for current agency policy regarding sampling and regulatory actions involving parasites in fish.
-
-
Multiple Sampling Plan (3 subsamples)
- (i) If no parasites are recovered in the first subsample, the lot is considered passable.
- (ii) If 1 to 5 parasites are recovered in the first subsample, examine the two additional subsamples.
- (iii) If 6 or more parasites are recovered in the first subsample, the lot is actionable.
- (iv) If the average number of parasites found in the 3 subsamples is less than 2 per kg, the lot is considered passable.
- (v) If the average number of parasites found in the three subsamples is 2 or more per kg, the lot is actionable.
- Report -- Report total number of parasites found per weight of sample(s) examined, and average number per kg. As appropriate, state identity of parasites.