Laboratory Information Bulletin (LIB) 4395: Analyses of Crystal Violet & Brilliant Green
Quantitative and Confirmatory Analyses of Crystal Violet (Gentian Violet) and Brilliant Green in Fish
Volume 23, May 2007
Wendy C. Andersen, Sherri B. Turnipseed, Christine M. Karbiwnyk, Rebecca H Lee, Susan B. Clark, W. Douglas Rowe, Mark R. Madson, Keith E. Miller+
U.S. Food and Drug Administration, Animal Drugs Research Center, Denver, CO
+University of Denver, Department of Chemistry and Biochemistry, 2190 E. Iliff Ave., Olin 202, Denver, CO 80208
The Laboratory Information Bulletin is a communication from the Division of Field Science, Office of Regulatory Affairs, U.S. Food and Drug Administration for the rapid dissemination of laboratory methods (or scientific regulatory information) which appear to solve a problem or improve an existing problem. In many cases, however, the report may not represent completed analytical work. The reader must assure, by appropriate validation procedures, that the reported methods or techniques are reliable and accurate for use as a regulatory method. Reference to any commercial materials, equipment, or process does not, in any way, constitute approval, endorsement, or recommendation by the U.S. Food and Drug Administration.
ABSTRACT
Liquid chromatographic methods are presented for the quantitative and confirmatory determination of crystal violet (CV; also known as gentian violet), leucocrystal violet (LCV), leucobrilliant green (LBG) and brilliant green (BG) in catfish. These methods are instrumental method modifications of published methods[1,2] for malachite green (MG) analysis to allow simultaneous determination of MG and CV. As in the MG method, residues were extracted from tissues with ammonium acetate buffer and acetonitrile, and isolated by partitioning into dichloromethane. LCV and LBG were oxidized to the chromic CV and BG by reaction with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. Samples were cleaned-up by solid phase extraction with alumina and propylsulfonic acid phases. Extracts were analyzed for total CV and BG by liquid chromatography with visible detection (LC-VIS) at 588 and 627 nm, respectively. A C18 column and gradient elution program was used for separation. The method was validated for catfish spiked with LCV over the range 0.25 to 10 ng/g and CV at 2 ng/g. Average recoveries were 90.6% (± 8.1% RSD, n = 45) for LCV and 84.4% (± 4.2% RSD, n = 6) for CV. The average recovery for BG/LBG over the range 0.5 to 10 ng/g was 67.2% (± 14.8% RSD, n = 31). CV and BG were confirmed in fish extracts by ion trap LC-mass spectrometry (LC-MSn) with no discharge-atmospheric pressure chemical ionization (ND-APCI). Average LC-MSn recoveries were 96.5, 96.6, and 70.2% for CV, LCV, and BG/LBG. The limit of detection for CV, BG, and MG varies from 0.25 to 0.5 ppb for the two different instrumental methods.
INTRODUCTION
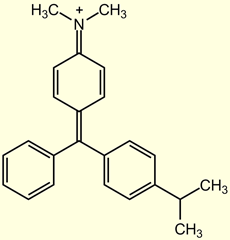
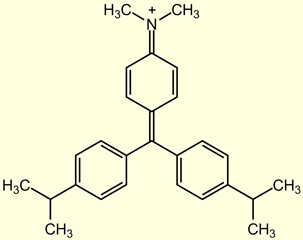
In the past five years, there have been many reports of the inappropriate use of malachite green (MG) as a veterinary drug to treat aquacultured fish. Malachite green is a well known and inexpensive dye effective against fungal and parasite infections in fish. The toxicity of malachite green is such that many countries, including the U.S., Canada, and the European Union, have banned the use of this dye in fish raised for human consumption. Crystal violet (CV), sometimes referred to as the medicinal preparation gentian violet, and brilliant green (BG) (Figure 1) are two other dyes in the triphenylmethane family that have antifungal properties similar to those of malachite green.[3] Like MG, CV is readily absorbed into fish tissue from water exposure and is reduced metabolically by fish to the leuco moiety, leucocrystal violet (LCV).[4] Crystal violet is also mutagenic and is not approved for use in aquaculture. The chemical similarity of BG to MG indicates that it would be comparably toxic to humans and effectively absorbed by fish. Though MG residues are now routinely monitored for by the FDA and many other international agencies, the potential for the continued misuse of triphenylmethane dyes is high, as MG, CV, and BG products are all advertised and available for the treatment of ornamental fish.
In the current study, our previously published malachite green residue method was adapted to simultaneous quantify and confirm the presence of CV and BG residues in catfish tissue. Sample extracts were oxidized with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone to convert leuco compounds to the colored, charged form of the compound for quantitative analysis by LC-VIS and residue confirmation by no-discharge APCI LC-MSn. Incurred samples of catfish exposed to CV and BG were also evaluated in this study.
EXPERIMENTAL
Equipment and reagent sources have been provided for information and guidance. Equivalent products may be substituted as appropriate.
Equipment
- Liquid chromatograph - Agilent model HP1100 series II with programmable diode array detector (DAD) (Avondale, PA). Operating conditions: 1.0 mL/min mobile phase flow rate; 35 °C column temperature; 65-140 bar column pressure; 100 µL volume injected. The DAD was equipped with a tungsten lamp and was set to monitor three absorption wavelengths, 588 nm for CV, 618 nm for MG, and 627 nm for BG (all are Δλ=4.0 nm; reference=725 nm, Δλ=8.0 nm).
- Liquid chromatograph mass spectrometer - Agilent (Avondale, PA) 1100 LC interfaced to a ThermoElectron (San Jose, CA) Finnigan DECA-XP Plus Ion Trap MS with an atmospheric pressure chemical ionization (APCI) source. XCaliber (Version 1.3) was the software used to operate both instruments.
- LC-VIS LC column - Alltech Alltima C18, 3 µm, 150 x 4.6 mm (P/N 81385), with guard cartridge (5 µm, 7.5 x 4.6 mm, P/N 96080) of the same phase (Alltech Associates, Deerfield, IL).
- LC-MS column - YMC phenyl 3-4-5 cartridge column, 3 µm, 120 Å, 50 x 4.0 mm (P/N PH12S030504WTA) (Waters Corp., Milford, MA), with a guard cartridge insert (20 x 4.0 mm) of the same phase.
- Blender/homogenizer - RobotCoupe Blixer, homogenizer, 4 quart, model RS1BX4V (RobotCoupe USA, Inc., Ridgeland, MS).
- Vortex Mixer - Vortex Genie 2, (Scientific Industries, Bohemia, NY).
- Centrifuge - refrigerated to 0 °C, capable of accelerating 50 mL tubes to 4000 rpm.
- Rotary evaporator - Büchi model R-110 with cold trap, evaporation temperature 50 °C (Brinkmann Instruments, Inc. Westbury, NY).
- Alumina (ALN-SPE) columns - Waters neutral alumina SPE columns, 6 mL, 1 g, 50-300 µm, P/N WAT054575 (Waters Corp., Milford, MA) were used for this study. In earlier malachite green work, Bakerbond alumina SPE columns (P/N 7214-07, JT Baker Inc., Phillipsburg, NJ), were used exclusively; however, the Bakerbond columns were not available for this study.
- Propylsulfonic acid (PRS-SPE) columns - Bond Elut LRC propylsulfonic acid solid phase extraction columns, 500 mg, disposable, P/N 1211-3038, with LRC 10 mL column connection adaptors, P/N 1213-1003 (Varian Inc., Palo Alto, CA.)
- SPE manifold - commercial SPE vacuum elution manifold with water aspirator and vacuum gauge.
- Centrifuge tubes. - 50 mL disposable, conical, graduated, polypropylene tubes with cap; 15 mL disposable, conical, graduated, polypropylene tubes with cap.
- Volumetric glassware and pipettes - 100.0 and 50.0 mL volumetric flasks, class A; 15 mL graduated centrifuge tubes with glass stoppers; adjustable volume pipettors with disposable polypropylene tips - 10-100 µL (Eppendorf, Brinkmann Instruments, Inc., Westbury, NY), 200-1000 µL (Ulster Scientific, Inc., New Paltz, NY), and 1-5 mL (Wheaton Science Products, Millville, NJ).
- Glassware - 150 mL pear-shaped boiling flasks with 24/40 necks and glass stoppers; 250 mL separatory funnels with PTFE stopcocks and glass stoppers 22; disposable Pasteur pipettes; 2 mL glass LC vials with caps.
Reagents
Reference standards - all reference standards were obtained from Sigma-Aldrich (St. Louis, MO). The crystal violet (CV) chloride salt used in this study, was identified as "gentian violet", to indicate that it met USP specifications for purity (97.5% by assay), (CI 42555), FW = 407.98 (CAS 548-62-9; G2039); leucocrystal violet (LCV), FW=373.53, (CAS 603-48-5; 219215); brilliant green (BG), (CI 42040) as the hydrogen sulfate salt, FW = 482.65 (CAS 633-03-4; B6756); malachite green (MG), (CI 42000) as the oxalate salt, FW = 929.0 (CAS 2437-29-8; M-6880); leucomalachite green, (LMG) FW=330.48, (CAS 129-73-7; 12,5660).
- Solvents - high purity chromatographic and spectrophotometric grade acetonitrile and methanol were used. Dichloromethane was liquid chromatographic grade. All water used was deionized and purified to 18.2 MΩ•cm (Millipore, Bedford, MA).
- Acetic acid - glacial, ACS grade, aldehyde-free, (CAS 64-19-7).
- Ammonium acetate - anhydrous, 98% purity, FW = 77.08 (CAS 631-61-8).
- 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone, (DDQ)- 98% purity, FW = 227.01 (CAS 84-58-2).
- 0.01 M DDQ stock solution: weigh 0.227 g DDQ into 100 mL volumetric flask and dilute to volume with acetonitrile; solution may be stored tightly capped and stored in the refrigerator for up to one month.
- 0.001 M DDQ working solution: pipette 5 mL of the 0.01 M DDQ solution into a 50 mL volumetric flask and dilute to 50 mL with acetonitrile; this solution was stored at room temperature and prepared fresh weekly.
- Diethylene glycol (DEG) - reagent grade (CAS 111-46-6).
- (Alumina - chromatographic grade, 80-200 mesh (CAS 1344-28-1) (AX0612-1, EM Science, Gibbstown, NJ).
- Hydroxylamine hydrochloride (HAH) - ACS reagent grade (99.8%), crystalline, FW = 69.49 (CAS 5470-11-1); 0.25 g/mL solution: weigh 25.0 g HAH into a 100 mL volumetric flask and dilute to mark with DI water.
- p-Toluene sulfonic acid (p-TSA) - 98.5%, monohydrate, FW 190.22 (CAS 6192-52-5); 1 M solution: weigh 9.5 g p-TSA into a 50 mL volumetric flask and dilute to mark with DI water.
- Formic Acid - ACS grade, 96%, (CAS 64-18-6); 0.1% solution: pipette 1 mL into a 1000 mL graduated cylinder and dilute to mark with DI water.
- Ammonium acetate buffer - Prepare a 0.1 M ammonium acetate solution by dissolving 7.7 g ammonium acetate in 1000 mL of DI water. To this solution, 8 mL of acetic acid and 5 mL of 1 M p-TSA were added, resulting in a final pH of 4.5. This buffer was used to prepare mobile phase A, as described in (l).
- Mobile phase A - 1:1 mixture (by volume) of ammonium acetate buffer and acetonitrile (filtered through a 0.45 µm PVDF membrane before use). Mobile phase A is used in this method for the LC-VIS chromatography, to dilute calibration standards and to elute SPE columns.
Leucobrilliant Green (LBG) Synthesis
LBG is not a commercially available product. LBG was prepared in solution, but as will be discussed later, it could not be isolated or analyzed by LC-VIS or LC-MS without reverting to BG. The synthesis method was modified from that of Pobiner and Hoffman.[5] To prepare a 100 µg/mL solution of LBG, 5.0 mg of BG (7.5 mg of BG hydrogen sulfate salt, 93.5% purity by elemental analysis) was added to a 100 mL beaker and dissolved in 7.5 mL of methanol. BG was reduced to LBG by adding 37 mL of water and 750 mg of potassium sulfite to the solution and mixing it by swirling the contents of the beaker until the dark green solution turned colorless. The solution was transferred to a low-actinic 50 mL volumetric flask and diluted to the mark with water. Although, Pobiner and Hoffman state that the LBG solution is stable for more than 4 months, the LBG stock solution prepared in our laboratory was used within 10 days of preparation. This 100 µg/mL stock solution was used to prepare LBG fortification and calibration solutions in methanol as described below.
Standard Preparation
-
Stock Solutions: 100 µg/mL. A sufficient amount of each of the reference standards was weighed into separate 100 mL volumetric flasks and diluted to volume with methanol to produce stock solutions that accounted for both the purity of the purchased standard and any counter ions or dimmers (Chart 1). A second identical stock solution was prepared as the Initial Calibration Verification (ICV) for CV, BG, and MG. Stock solutions (and Stock-ICVs) were stored in glass protected from light at room temperature, and generally prepared every six months. The BG stock solution was not evaluated for stability beyond two months. LCV and LMG solutions were stored in low-actinic glass.
Chart 1 To prepare a 100 µg/mL stock solution that contains 10.0 mg of: Correct for: By adding this amount of the compound to 100.0 mL of methanol Crystal Violet (CV) Standard purity (97.5% specified) and chloride counter ion 11.23 mg Leucocrystal violet (LCV) Standard purity (99.9% EA) 10.01 mg Brilliant Green (BG) Standard purity (93.5% EA) and hydrogen sulfate counter ion 13.39 mg Malachite Green (MG) Standard purity (99.3% EA), oxalate counter ion, and dimer 14.20 mg Leucomalachite Green (LMG) No correction used; purity was above 99.9% (EA) 10.00 mg EA = Elemental Analysis; Purity was calculated from the elemental analysis supplied from the Sigma-Aldrich Certificate of Analysis.
- Intermediate Solutions (IS): The following chart (Chart 2) outlines the intermediate solutions and standards that were used in this study to prepare calibration standards and to spike fish tissue to prepare fortified samples. However, for normal regulatory analysis, analysts will typically only prepare fortified tissue samples at the 1 ng/g concentration level of LCV, LMG, and/or BG, and will typically prepare a calibration curve that covers the range of 0.5 to 4 ng/mL of CV, MG, and/or BG. Analysts may find it practical to prepare different volumes or concentrations of the actual intermediate solutions to more appropriately address a smaller concentration range of calibrants and spiking solutions, or to prepare intermediate solutions that combine dyes.
Chart 2 Intermediate Solution Concentrations
used in this studySolution Preparation IS1 - 1.0 µg/mL 1.0 mL of Stock diluted to 100.0 mL with methanol IS2 - 0.1 µg/mL 1.0 mL of IS1 diluted to 10.0 mL with methanol IS3 - 0.025* µg/mL 1.0 mL of IS2 diluted to 4.0 mL with methanol * Only used to prepare the lowest concentration standards and fortifications used in method detection limit determinations.
CV, BG, and MG solutions were prepared in duplicate, with the second solution designated as the ICV. All solutions were stored in glass and kept in a dark cabinet at room temperature when not in use. Leuco solutions were additionally stored in low-actinic glass. IS1 solutions were prepared monthly; IS2 and IS3 solutions were prepared weekly. Calibration standards were prepared every one to two days. The following chart (Chart 3) is a useful guide for preparing calibration standards and fortified samples:
Chart 3 Pipette Dilute to 5.0 mL with mobile phase A to prepare calibration standard with concentration: Add to 5.0 g of ground fish tissue to prepare fortified sample with concentration: 100 µL of IS1 20 ng/mL 50 µL of IS1 10 ng/mL 10 ng/g 200 µL of IS2 4 ng/mL 4 ng/g 100 µL of IS2 2 ng/mL 2 ng/g 50 µL of IS2 1 ng/mL 1 ng/g 25 µL of IS2 or 100 µL of IS3 0.5 ng/mL 0.5 ng/g 50 µL of IS3 0.25 ng/mL 0.25 ng/g 30 µL of IS3 0.15 ng/mL
Sample Preparation
Skinless, aquacultured fillets of fresh catfish were obtained from a local market, and cut into 3 to 5 cm cubes. Muscle tissue was blended with dry ice in a blender/homogenizer with pulsed action until contents were uniform and had the consistency of a fine powder. Homogenate was placed in a Whirl-Pak bag, loosely sealed and stored in the freezer overnight to allow the carbon dioxide to dissipate, then sealed until the time of analysis.
Sample Fortification
Portions (5.0 g) of thawed tissue composite were fortified according to Chart 3. Samples were allowed to sit at room temperature for at least 15 minutes before proceeding with the extraction. For the determination of crystal violet residues, tissue samples were fortified at concentrations of 0.25, 0.5, 1.0, 2.0, 4.0, and 10.0 ng/g of LCV and 2.0 ng/g of CV. For brilliant green, samples were fortified at concentrations of 2.0 ng/g of LBG and 0.5, 2.0, and 10.0 ng/g of BG. For malachite green, fortified samples were only prepared with the concentration of 0.5 ng/g of LMG to establish the method detection limit; a complete validation data set for MG can be found elsewhere.[1,2]
Extraction
The extraction method detailed herein is identical to that reported in LIB 4363, and can be used for the simultaneous analysis of crystal violet, brilliant green, and malachite green.[1,2] Accurately weigh 5.0 g tissue composite into a 50 mL centrifuge tube and let thaw. Add 5 mL of ammonium acetate buffer, 1 mL of HAH solution and 100 µL of p-TSA solution. Cap the centrifuge tube and mix by vortexing vigorously for 30 seconds. Add 25 mL of acetonitrile, cap and shake vigorously for 30 seconds. Add 10 g of alumina, cap and shake vigorously for 15 seconds. Centrifuge at 0 °C for 5 minutes at 4000 rpm (2730 rcf).
Add 50 mL water and 2 mL DEG to a 250 mL separatory funnel. Decant the sample supernatant into the separatory funnel. Add another 25 mL of acetonitrile to the solids remaining in the 50 mL centrifuge tube, cap, mix by vortexing for 30 seconds, and then shake vigorously for 30 seconds. Centrifuge at 0 °C for 5 minutes at 4000 rpm. Decant supernatant into original 250 mL separatory funnel containing first extract. Add 25 mL dichloromethane to separatory funnel, stopper, invert and open stopcock to release pressure. Close stopcock and extract by mixing funnel for 30 seconds. Allow phases to separate for no more than 10 minutes, then drain the lower dichloromethane layer into a 150 mL pear-shaped boiling flask. Add an additional 25 mL of dichloromethane to the separatory funnel, and repeat the liquid extraction as before. Collect the organic phase in the original 150 mL boiling flask, combining the first and second extracts.
Rotoevaporate the contents of the boiling flask just to dryness under reduced pressure while heating flask in a water bath set at 50 °C. Add 3 mL of acetonitrile to the oily residue and swirl to dissolve. At this point, sample may be stoppered and stored overnight at room temperature and protected from light. Add 3 mL of 0.001 M DDQ solution and swirl to mix. Sample will immediately change from orange to a dark red-purple color. Allow oxidation reaction to proceed for 30 minutes, with periodic sample agitation. The color of the sample may lighten over time; however, if the sample pales significantly or clears, a lower than expected recovery might be obtained.[6]
While the oxidation reaction is occurring, condition disposable ALN-SPE and PRS-SPE columns with 5 mL of methanol followed by 5 mL of acetonitrile. Add an additional 5 mL of acetonitrile to the PRS-SPE to serve as a solvent reservoir. Position the ALN-SPE column above the PRS-SPE column using a column adapter and insert the SPE in a vacuum elution system. Transfer the oxidized sample solution to the ALN-SPE, and adjust vacuum to allow sample to elute onto the PRS-SPE column at a drop rate of approximately 4 mL/min. Wash boiling flask with two sequential portions of 5 mL acetonitrile and add each wash to the ALN-SPE just as the previous liquid portion clears the upper column. Remove the ALN-SPE column and discard. Wash the PRS-SPE with 5 mL acetonitrile. Pull vacuum on the PRS-SPE column for 2-3 seconds to remove most of the residual acetonitrile (column should not be fully dried). Remove PRS-SPE from the vacuum elution system and elute by gravity into a 15 mL graduated centrifuge tube with 4 mL of mobile phase A. With hand bulb or syringe, blow out remaining solvent from column into centrifuge tube. Dilute sample to 5.0 mL with mobile phase A, and transfer a portion of the sample to a chromatographic vial for LC-visible analysis. The remaining portion of this sample may be transferred to a second chromatographic vial for simultaneous confirmation by ND-APCI LC-MSn or stored in the refrigerator (ca. 4 °C) for a couple days for later residue confirmation. Saved samples should not be frozen. In this study, extracts were typically analyzed by LC-MSn within 24 hours of the extraction. One set of standards and samples fortified with LCV at 0.5 ng/g was analyzed by LC-MSn 8 days after the extraction; the results are consistent with the LC-VIS analysis that was done on the same day.
Quantification by LC-VIS
The LC was operated with a mobile phase flow rate of 1.0 mL/min and column temperature of 35 °C. All injections were 100 µL and a needle wash of mobile phase A was used. The DAD was set to monitor absorbance at 588 nm for crystal violet, 618 nm for malachite green, and 627 nm for brilliant green (725 nm reference for all) using a tungsten lamp. Mobile phase A is a 1:1 mixture (by volume) of ammonium acetate buffer and acetonitrile (filtered through a 0.45 µm PVDF membrane before use). Mobile phase B is acetonitrile. The LC-VIS mobile phase elution profile is isocratic 95% A (equivalent to 47.5% ammonium acetate buffer and 52.5% acetonitrile) for the first 8 minutes, gradient to 50% A from 8 to 16 minutes, and gradient back to the initial composition from 16 to 18 minutes.
Leuco compounds are quantitatively oxidized to the dyes by reaction with DDQ during the extraction. Therefore, although samples were fortified with the leuco moiety, the recovery of the leuco compound was calculated based solely on the recovery of the dye, using the calibration curve generated for the dye. After injecting all of the calibration standards and samples, the 1 or 2 ppb standard should be re-injected as the Continuous Calibration Verification (CCV). The 2 ppb ICV standard should be within ± 15% of the peak area counts of the 2 ppb standard and CCV should be within ± 15% of the peak area counts of the initial injection of that standard. After the injection of each set of standards and samples, or at the end of each day, the chromatography column should be flushed with 100% methanol for at least 30 minutes.
Residue Confirmation and Quantification by ND-APCI LC-MSn
The LC-MS was tuned by flowing a standard solution (0.5 µg/mL of dye in 50/50 water/methanol) at a rate of 10 µL/min using a syringe pump into a stream of 700 µL/min 63/37 0.1% formic/acetonitrile via a T fitting. Source parameters such as lens voltages, gas flows, vaporizer and capillary temperatures, and collision energies were optimized in this manner. Typical MS parameters for no-discharge APCI were determined to be: corona discharge 0 µA; vaporizer temperature, 400 °C; capillary temperature, 220 °C; capillary voltage, 14V; sheath gas, 60; auxiliary gas, 40. The number of prescans was equal to two, and the maximum inject time was set to 500 ms for MS2 scans. The MS acquisition program consisted of MS2 scans of m/z 372, 385, and 329 for CV, BG, and MG, respectively. The collision energy was 55 for CV and BG and 50 for MG. The range was m/z 150-400 for CV and BG, and m/z 150-350 for MG. For all three compounds, the isolation width was 2 amu, Q was 0.25, and the activation time was 30 ms.
The final instrument procedure consisted of a two-stage isocratic LC program consisting of 0.1% formic acid and acetonitrile (63% / 37%) from 0-7 minutes and stepped to a 55% / 45% composition from 7.1-15 minutes. The column was washed with 100% ACN from 15.1-17 minutes, and then the mobile phase composition was ramped back to the initial conditions for a total run time of 20 minutes. The column oven was maintained at 30 °C. The mobile phase flow was 700 µL/min. Ten microliter injections were made with a needle wash of water or methanol. The divert valve was switched from waste to the MS at 1 minute.
The treatment of the data varied depending on whether qualitative (confirmatory) or quantitative data were being evaluated. For qualitative assessment, individual ion transition chromatograms (crystal violet: m/z 372, 356, 251, and 328; brilliant green: m/z 385, 355, 341, and 236; malachite green: m/z 329, 313-315, 284-286, 251, and 208) were generated and the resulting chromatographic peaks were integrated with the ICIS algorithm in the Qual Browser® software program. A Gaussian smoothing function of five points was applied. Relative abundances were calculated from these peak areas and compared to contemporary standards. For quantitative assessment the area counts of the CV peak from the total ion chromatogram of the m/z 372 product ion scan, not the extracted ion chromatograms, were used. Peak areas were calculated by the Quan Browser® software program and the ICIS integration program was selected for the processing method. A calibration curve was generated with the same standards as were used for the LC-VIS analyses.
RESULTS AND DISCUSSION
In this Laboratory Information Bulletin, the previously published malachite green method[1,2] was expanded to include crystal violet and brilliant green. During the development of this method, negative control and method blank samples were analyzed and consistently found to have low levels of crystal violet contamination in the range of 0.4 to 0.8 ppb. An effort was undertaken to meticulously clean all glassware and laboratory equipment between analyses; however, the background level of CV was persistent. Only when the laboratory work areas were scrupulously cleaned was it realized that the electrostatic nature of CV is such that simply opening the bottle and weighing the compound to prepare standards is enough to scatter CV across the lab where it easily adheres to the scale, lab bench, lab coats, gloves, pens, glassware, etc. Great caution must be taken to thoroughly decontaminate the work space before preparing samples. Even with such precautions, 2 of the 12 negative controls were found to be positive for CV by LC-MSn, although the levels were below 0.1 ppb.
The method requires approximately 5 hours to extract 6-10 samples and to prepare calibration standards. The solvent evaporation step is the most time consuming, requiring approximately 15 to 20 minutes to evaporate each sample. Alternative evaporation schemes were investigated to reduce the time required for evaporation. In one scenario, the two dichloromethane extracts were collected in a 250 mL polypropylene centrifuge bottle, and this volume was reduced using an N-evap nitrogen evaporator that could accommodate multiple samples at one time. About 1.25 hours were required to evaporate the sample, which contained a volume of approximately 75 mL. This option makes sense when more than 5 samples are analyzed at once; however, the additional cost of disposable centrifuge bottles must also be considered, and the spatial orientation of the evaporator must be able to accommodate enough of the large bottles to be advantageous. A second evaporation scheme was investigated wherein organic extracts were evaporated in two portions in 50 mL centrifuge tubes. This required multiple sample transfers and tube rinsings, and furthermore, required about 2.5 hours to evaporate a 75 mL extract. Although the tubes cost less than the centrifuge bottles, this option did not appear to provide any advantages over rotary evaporation of one sample at a time. The best evaporation scheme appears to be using two or more rotary evaporation units at a time to increase sample throughput.
Leucobrilliant Green Synthesis
Leucobrilliant green is not available as a commercial product. Few methods have described in detail the synthesis of LBG. One author summarizes that LBG could be formed from the condensation of benzaldehyde with diethylaniline in acid.[7] Another refluxed BG with sodium bisulfite for 21 days to produce a black oil. The product was purified to yield a thick colorless oil that turned green, presumably converting back to BG.[8] Pobiner and Hoffman described a method to produce LBG from the reduction of BG with sodium sulfite in a methanol-water solution.[5]
Our synthesis was based on the Pobiner and Hoffman method with several deviations, including the use of potassium sulfite in place of sodium sulfite. Attempts to isolate LBG from the K2SO3 solution were made by extracting LBG into diethyl ether, and then trying various techniques to exchange the LBG into a solvent suitable for use in the LC-VIS and LC-MSn analytical methods. Evaporation of the ether resulted in a green-blue residue, indicating that the colorless LBG had re-oxidized to BG. The addition of acetonitrile to the ether extract, followed by evaporation of most of the ether resulted in a clear acetonitrile extract. Addition of 1% formic acid, water, or methanol to the acetonitrile extract resulted in solutions that immediately (1% formic acid) or slowly (over 20 minutes, water and methanol) turned pale blue-green (water and methanol). Solutions of ACN-MeOH and ACN-H2O were quickly infused into the ND-APCI source of the MS, and the resulting spectrum only showed the presence of BG. An LBG-K2SO3 solution was also directly infused into an electrospray source on the MS, and only BG was detected. Finally, a fish sample was spiked with a high concentration (100 µg) of the LBG-K2SO3 solution to determine if LBG could be isolated as a fish extract in the absence of DDQ oxidation. Less than 5 minutes after the extraction began, the supernatant began to turn blue-green, indicating that LBG is not stable under the extraction conditions. The following section describes LC-VIS experiments wherein standards of LBG-K2SO3 were injected, and BG was detected, indicating that LBG is also not stable under the chromatographic conditions of this method.
Residue Quantification by LC-VIS
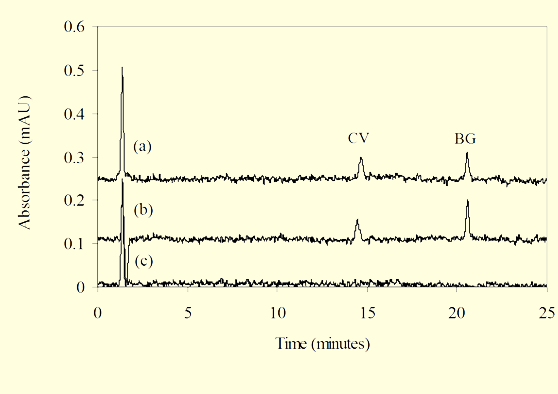
Table 1 lists recoveries of residues for catfish fortified with LCV, CV, LBG, BG, and MG. Typical chromatograms are shown in Figure 2 for 0.5 ng/g CV and BG standards, catfish fortified with 0.5 ng/g LCV and BG, and control catfish. The day to day reproducibility of the method was measured by extracting five fish samples fortified with 2 ng/g of LCV and with 2 ng/g of BG on each of three days; LCV inter-day data is shown in Table 2.
A calibration curve was generated daily from the peak area response of at least six dye standards with concentrations typically 0, 0.5, 1.0, 2.0, 4.0, and 10.0 ng/mL. Additional bracketing standards were injected for samples that were fortified at concentrations of 0.25, 0.5, and 10.0 ng/g, as appropriate. For regulatory purposes, the fortification level should be the level of concern, which is currently 1.0 ng/g; therefore, when analyzing regulatory samples, a calibration curve consisting only of the 0, 0.5, 1, 2, and 4 ng/g standards is appropriate. Correlation coefficients (r2) for acceptable standard curves were at least 0.995. Typical peak area responses for the 2 ng/g calibration standards were 3.1, 3.0, and 2.6 mAU•s for CV, BG, and MG, respectively. Retention times ranged from 8.0-9.6, 14.5-16.3, and 17.7-20.3 minutes for MG, CV, and BG, respectively, with times increasing with increasing age of the column.
Residue Confirmation and Quantification by ND-APCI LC-MSn
The three dyes are charged (not protonated) species in solution. Molecular ions are m/z 372 for CV, m/z 385 for BG, and m/z 329 for MG. The product ions for the three dyes are shown below in Chart 4. High collision energies were required to obtain significant abundance of these ions. The quantity of the dyes that can be detected and yield an adequate product ion spectrum is less than 2 pictograms.
| Ion corresponds to | MG (m/z) | CV (m/z) | BG (m/z) |
|---|---|---|---|
| M+ | 329 | 372 | 385 |
| [M- CH3]+ | 314 | 357 | |
| [M- H-CH3]+ | 313 | 356 | |
| [M- H-C2H5]+ | 355 | ||
| [M- NC2 H6 ]+ | 285 | 328 | 341 |
| [M- C6 H6 ]+ | 251 | ||
| [M- C6 H5 -CH3 ]+ | 237 | ||
| [M- NC2 H5 -CH3 ]+ | 314 | ||
| [M- NC8 H11 ]+ | 251 | ||
| [M- NC10 H15 ]+ | 236 |
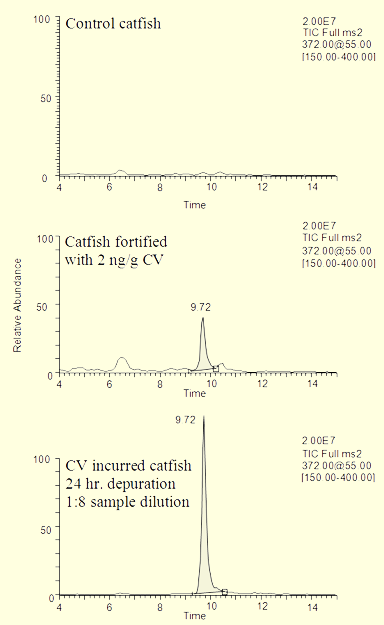
The LC-MSn method was developed to provide confirmation of the analysis of the dye residues. In order to achieve the most efficient use of laboratory resources, the same extracts prepared for the LC-VIS method were used for LC-MS analysis. In most cases, sample extracts were refrigerated overnight and analyzed the following day by LC-MSn. As shown in Table 1, residues were confirmed in all fortified samples according to CVM confirmation criteria.[9] This guidance states that the retention time must be within 5% of that of a standard, that the product ion spectra must be visually similar to a standard with a minimum of unexplained background ions, and that the relevant product ions for the residue must be present at an appreciable level and at the correct relative abundance as compared to a standard compound run that day. While this information also could have been obtained from the MS2 scan, extracted ion chromatograms were generated for each product ion in order to consistently determine relative abundance ratios. The peaks at the corresponding retention time for the dyes in these extracted ion chromatograms were integrated, and the areas obtained were compared to that for the largest ion in the product spectrum (eg. m/z 356 for CV, m/z 341 for BG, and m/z 329 for MG). Examples of the MS2 total ion chromatograms obtained from the CV analyses of catfish samples (extracts from control, 2 ng/g fortified, and incurred fish) are shown in Figure 3, along with extracted ion chromatograms and the MS2 spectrum from catfish fortified at 0.5 ng/g. Similar data for BG analysis are illustrated in Figure 4.
Tables 3 and 4 consist of a summary of retention time and relative abundance data obtained for catfish spiked with LCV and BG/LBG. For any one day's analysis, the variance of the retention times and relative abundances is much less than what is required by the confirmation criteria. Although the relative abundances varied over the six-month study, the data obtained from the extracted samples matched that of solvent-based standards analyzed on the same day. For brevity, the qualitative results are presented as averages for each type of sample analyzed in a day. It is important to note, however, that each individual sample was evaluated to determine if confirmation criteria were met.
It is evident from the relative abundance data that CV and BG were confirmed in all fortified samples. BG was not confirmed in any of the catfish control tissue extracts analyzed; however, CV was confirmed in two of the control extracts generated for this validation study at a level that was less than 0.1 ng/g. Residues were not present in any of the solvent blanks (injected between standards and fish extracts in every LC-MSn sequence), indicating that instrument carry-over was not an issue. Also, residues were not found in method reagent blanks.
The LC-VIS method was intended to be the primary method to routinely screen and quantify dye residues in numerous laboratory fish samples, without straining the resources of high-demand LC-MS instruments. However, a comparative analysis of quantification data by both the LC-VIS and the LC-MSn methods was also included in this study. The data for the recovery of LCV, CV, LBG, BG, and LMG from tissues using LC-MSn with no-discharge APCI are shown in Table 1. Percent recovery was determined by measuring the amount of the dye in the sample (peak area from the total ion chromatograms, not the extracted ion chromatograms) and comparing this amount to a calibration curve generated using standards prepared according to Chart 3. The standard curves were linear (r2 > 0.995) in the range of 0.5 to 10 ng/g. For low concentration samples, (1 ng/g or lower), quantification was obtained by limiting the calibration range to 0.5 to 4 ng/g, and often adding lower concentration standards to better bracket the concentration of the fortified sample. Although the precision for the LC-VIS determination was typically better than for the LC-MSn method, the two detection methods generally produced comparable results with average recoveries equivalent within the margin of error (RSD) for each method. For example, the overall average recovery of LCV and CV from 51 fortified samples was 89.9% (8.1% RSD) for LC-VIS and 96.5% (10.2% RSD) for LC-MSn. These results indicate that LC-MSn is an acceptable alternative quantification method offering advantages for check analyses.
Metabolic Marker Residues
The emphasis of the validation study was to determine the leuco residues since these metabolites are the major compound found in tissue.[4] In our previous studies, the recovery of LMG was found to be significantly higher than that of MG (e.g., 64% MG and 89% LMG for samples of 2 ng/g fortified catfish).[2] In the current study, the recovery of CV was found to be comparable to that of LCV (Table 1); however, it is clear from the work of Thompson and coworkers that LCV is the appropriate marker residue for fish that have been treated with CV. [4] In that study, 90 catfish were exposed to a water bath containing 100 ng/mL CV for 1 hour. The fish were then placed in clean water and sampled at regular intervals. One hour after CV exposure, the catfish tissue contained 11.7 ng/g of LCV and only 0.5 ng/g of CV. After 4 hours and up to 79 days, the LCV concentration ranged from 16 to 1.5 ng/g, while the CV concentration was generally undetectable.
The case of evaluating brilliant green exposure is not as straightforward. While it is reasonable to assume that fish would metabolize BG similarly to CV and MG considering the structural similarities of the compounds (Figure 1), we have not been able to directly analyze LBG before it converts to BG. For this reason, and because we could not produce a pure LBG standard, the majority of the validation data in this study was produced using spikes of BG. The recovery of BG from catfish fortified with 2 ng/g of BG was 64% (14% RSD, n = 17, LC-VIS), which is comparable to the 64% recovery found for catfish fortified with MG. One set (n=5) of samples was prepared wherein catfish samples were fortified with 2 ng/g of the potassium sulfite solution of LBG. When the recovery was calculated using a BG calibration curve, the average recovery of the sample was 59.5% (4.0% RSD, LC-VIS), consistent with the BG spikes. When the recovery was calculated using an LBG-based calibration curve, the average recovery of the sample was 71.9% (3.5% RSD, LC-VIS). It should be noted that in the latter case, the calibration standards were prepared from the LBG-K2SO3 stock solution, but the resulting chromatogram showed BG peaks. Additional studies confirmed that LBG-K2SO3standards produced a BG peak that was 22.3% lower than the BG standards. The size of the BG peak produced from LBG standards did not change as a function of time, suggesting that the re-oxidation of LBG to BG does not go to completion. Until BG metabolism studies in fish are undertaken and/or LBG can be better stabilized, we suggest that BG should be used as the marker residue for fish that have been treated with BG.
Incurred Residues
In addition to the fortified samples, several catfish (Ictalurus punctatus) were dosed with crystal violet and brilliant green and analyzed by both instrumental methods. Fish were exposed to water baths containing 50 ng/mL CV or BG for 1 hour, then were removed to clean water and sampled 0 to 24 hours after exposure. Skinless fillets were ground with dry ice, extracted and analyzed as described above. The results of these incursion studies are summarized in Table 5. Higher concentration tissue extracts were appropriately diluted with Mobile Phase A to conform to the calibration range. The LC-VIS and LC-MSn results agreed very well with each other. Significantly higher tissue concentrations of CV were found in this study as compared with the results of Thompson and coworkers, where catfish were dosed at a higher level.[4]
Method Detection Level
To determine the method detection level (MDL) for this method, catfish samples (n = 7) were fortified with 0.25 ng/g LCV, 0.5 ng/g BG, or 0.5 ng/g LMG then extracted and analyzed. The MDL was calculated from the standard deviation of the calculated concentrations for the seven samples multiplied by the Student's t value at the 99% confidence level. From this data set, the MDL was found to be 0.07 ng/g for CV, 0.07 ng/g for BG, and 0.15 ng/g for MG for the LC-VIS method. The limit of quantification (LOQ) was ten times the standard deviation of the concentration of the seven replicates, or 0.18 ng/g for CV, 0.21 for BG, and 0.49 for MG using LC-VIS.
CONCLUSION
The preceding method enables the multi-residue determination of residues of malachite green, crystal violet (gentian violet), brilliant green, and their leuco metabolites in fish. Leuco metabolites were converted to the dyes using in-situ oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. Residues can be quantitatively determined using LC-VIS or ND-APCI LC-MSn. The limits of quantification were determined to be 0.18 ng/g for CV, 0.21 for BG, and 0.49 for MG using LC-VIS. ND-APCI LC-MSn provides residue confirmation at even lower levels for these residues.
ACKNOWLEDGEMENTS
We gratefully acknowledge Charles Gieseker and Renate Reimschuessel from the U.S. Food and Drug Administration Center for Veterinary Medicine for providing the incurred fish tissues used in this study.
REFERENCES
- W. C. Andersen, S. B. Turnipseed, and J. E. Roybal, "Quantitative and Confirmatory Analyses of Malachite Green and Leucomalachite Green Residues in Fish and Shrimp" Laboratory Information Bulletin 4363, U.S. Food and Drug Administration (2005).
- W. C. Andersen, S. B. Turnipseed, and J. E. Roybal, Journal of Agricultural and Food Chemistry, 54 (2006) 4517.
- D. J. Alderman, Journal of Fish Diseases, 5 (1982) 113.
- H. C. Thompson, Jr., L. G. Rushing, T. Gehring, and R. Lochmann, Journal of Chromatography, B 723 (1999) 287.
- H. Pobiner and H. T. Hoffman, Jr., Analytica Chimica Acta, 141 (1982) 419.
- W. C. Andersen, J. E. Roybal, and S. B. Turnipseed, Journal of the AOAC International, 88 (2005) 1292.
- F. J. Green, Ed. The Sigma-Aldrich Handbook of Stains, Dyes, and Indicators, Aldrich Chemical Company, Inc., Milwaukee, WI, (1990).
- R. N. Macnair, Journal of Organic Chemistry, 33 (1968) 1945.
- "Guideline for Industry: Mass Spectrometry for Confirmation of the Identity of Animal Drug Residues" Guideline #118 (PDF, 112Kb), U.S. Food and Drug Administration. (2003). http://www.fda.gov/cvm/guidance/guide118.pdf
| Concentration (ng/g) |
LC-VIS Average Recovery % (% RSD) |
Number of Samples by LC-VIS |
LC-MSn Confirmed/Analyzed |
LC-MSn Average Recovery % (% RSD) |
|---|---|---|---|---|
| 0.25 - LCV 0.5 - LCV 1.0 - LCV 2.0 - LCV 4.0 - LCV 10.0 - LCV |
82.1 (9.5) LOQ 93.1 (7.3) 86.8 (4.7) 94.0 (4.1) 96.7 (3.1) 99.8 (1.3) |
7 5 15 8 5 5 |
7/7 5/5 15/15 8/8 5/5 5/5 |
92.7 (9.2) 103.9 (3.0) 94.4 (7.7) 103.6 (12.3) 95.4 (14.7) 90.1 (4.9) |
| 2.0 - CV | 84.4 (4.2) | 6 | 6/6 | 96.6 (11.1) |
| 2.0 - LBG | 71.9 (3.5) | 5 | 5/5 | 80.5 (11.0) |
| 0.5 - BG 2.0 - BG 10.0 - BG |
.3 (5.5) LOQ 63.8 (13.6) 49.7 (0.0) |
7 17 2 |
7/7 17/17 2/2 |
79.7 (9.7) 65.7 (25.5) 48.8 (5.1) |
| 0.5 - LMG | 90.3 (10.3) LOQ | 7 | 7/7 | 82.3 (17.6) |
| Sample | Day 1 (LC-VIS/LC-MSn) |
Day 2 (LC-VIS/LC-MSn) |
Day 3 (LC-VIS/LC-MSn) |
|---|---|---|---|
| Spike 1 Spike 2 Spike 3 Spike 4 Spike 5 |
83.8 / 97.2 89.4 / 95.0 85.4 / 95.9 82.5 / 95.4 78.5 / 91.5 |
81.7 / 93.6 89.0 / 81.8 93.1 / 85.9 92.9 / 92.0 89.0 / 89.3 |
87.3 / 89.9 86.3 / 101.9 |
| Average % Recovery % RSD |
83.9 / 95.17 4.8 / 2.2 |
89.2 / 88.6 5.2 / 5.4 |
87.4 / 99.7 1.9 / 9.0 |
| Day (date) |
Description of Analysis | Retention Time (min.) |
% Relative Abundances | |||
|---|---|---|---|---|---|---|
m/z 356 |
m/z 372 |
m/z 327 |
m/z 251 |
|||
| Day 1 (8/29/06) | Average of Standards | 9.65 | 100 | 47 | 7 | 18 |
| Averagea of 1 ng/g LCV spikes | 9.61 | 100 | 42 | 6 | 20 | |
| Day 2 (8/30/06) | Average of Standards | 9.62 | 100 | 44 | 6 | 20 |
| Average of 2 ng/g LCV spikes | 9.61 | 100 | 43 | 5 | 20 | |
| Average of 4 ng/g LCV spikes | 9.60 | 100 | 42 | 6 | 19 | |
| Day 3 (9/01/06) | Average of Standards | 9.61 | 100 | 48 | 5 | 19 |
| Average of 10 ng/g LCV spikes | 9.61 | 100 | 48 | 5 | 20 | |
| Day 4 (10/12/06) | Average of Standards | 9.72 | 100 | 38 | 5 | 18 |
| Average of 0.5 ng/g LCV spikes | 9.65 | 100 | 36 | 6 | 18 | |
| Average of 1 ng/g LCV spikes | 9.66 | 100 | 38 | 6 | 18 | |
| Day 5 (10/13/06) | Average of Standards | 9.69 | 100 | 46 | 6 | 19 |
| Average of 0.25 ng/g LCV spikes | 9.66 | 100 | 42 | 7 | 22 | |
| Average of 1 ng/g LCV spikes | 9.66 | 100 | 40 | 5 | 20 | |
| Day 6 (1/12/07) | Average of Standards | 9.73 | 100 | 16 | 20 | 6 |
| Average of 1 hr Incurred | 9.74 | 100 | 18 | 19 | 6 | |
| Day 7 (1/18/07) | Average of Standards | 9.66 | 100 | 26 | 20 | 7 |
| Average of 24 hr Incurred | 9.72 | 100 | 21 | 16 | 5 | |
| Average of 0 hr Incurred | 9.31 | 100 | 20 | 17 | 7 | |
aSpikes and incurred are all (n = 5) unless indicated.
| Day (date) |
Description of Analysis | Retention Time (min.) |
% Relative Abundances | |||
|---|---|---|---|---|---|---|
m/z 341 |
m/z 385 |
m/z 355 |
m/z 236 |
|||
| Day 1 (1/10/07) | Average of Standards | 11.96 | 100 | 4 | 16 | 5 |
| Averagea of 1 hr Incurred | 11.72 | 100 | 5 | 15 | 6 | |
| Day 2 (1/11/07) | Average of Standards | 11.68 | 100 | 5 | 19 | 5 |
| Average of 24 hr Incurred | 11.65 | 100 | 6 | 20 | 7 | |
| Day 3 (2/28/07) | Average of Standards | 10.64 | 100 | 4 | 20 | 5 |
| Average of 2 ng/g LBG spikes | 10.63 | 100 | 7 | 16 | 5 | |
| Day 4 (2/28/07) | Average of Standards | 10.64 | 100 | 5 | 16 | 5 |
| Average of 2 ng/g BG spikes | 10.64 | 100 | 6 | 20 | 5 | |
| Day 5 (3/1/07) | Average of Standards | 10.62 | 100 | 5 | 19 | 4 |
| Average of 0.5 ng/g spikes | 10.63 | 100 | 6 | 14 | 5 | |
| Day 6 (3/5/07) | Average of Standards | 10.58 | 100 | 11 | 19 | 6 |
| Average of 2 ng/g spikes | 10.63 | 100 | 9 | 19 | 6 | |
| Average of 10 ng/g (n=2) | 10.63 | 100 | 9 | 16 | 7 | |
aSpikes and incurred are all (n = 5) unless indicated.
| Treatment and Depuration | Averagea Concentration found by LC-VIS ± RSD (ng/g) | Averagea Concentration found by LC-MSn ± RSD (ng/g) |
|---|---|---|
| CV, 0 hours | 17.8 ± 2.2 | 20.6 ± 7.9 |
| CV, 1 hour | 42.4 ± 3.2 | 40.8 ± 4.0 |
| CV, 24 hours | 80.8 ± 4.9 | 78.7 ± 11.8 |
| BG, 1 hour | 13.2 ± 14.3 | 11.4 ± 13.0 |
| BG, 24 hours | 15.3 ± 7.1 | 17.3 ± 9.7 |
aFive samples of each fish were extracted and analyzed.
| Malachite Green | Crystal Violet (Gentian Violet) | Brilliant Green |
|---|---|---|