Unauthorized E-cigarettes that Appeal to Youth
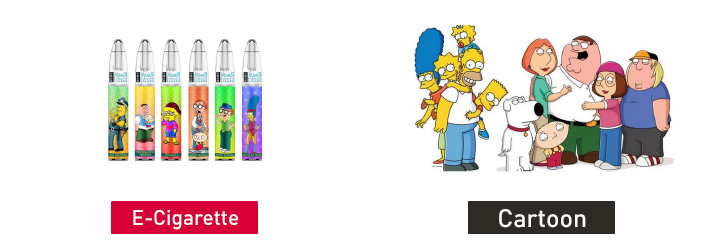
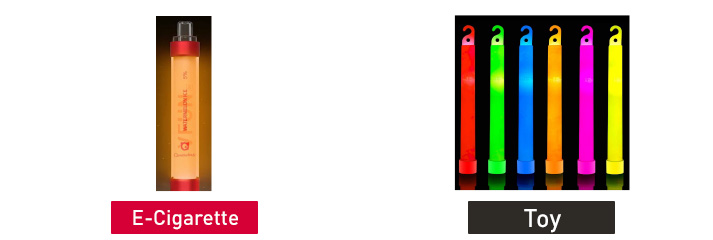
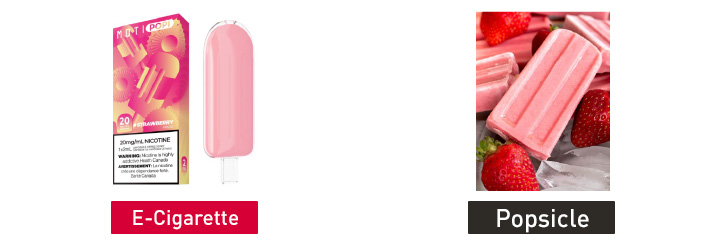
On Nov. 16, 2022, FDA issued warning letters to the following five firms for the unauthorized marketing of 15 different e-cigarette products. Each e-cigarette product is packaged to look like toys, food, or cartoon characters and is likely to promote use by youth. None of the manufacturers submitted a premarket application for any of the unauthorized products.
The FDA issued warning letters to:
- Wizman Limited doing business as Wizvapor
- Shenzhen Fumot Technology Co. Ltd., doing business as R and M Vapes
- Shenzhen Quawins Technology Co., Ltd.
- Ruthless Vapor
- Moti Global
The warning letters notify the recipients that e-cigarettes without a marketing authorization order are adulterated and misbranded, and that selling or distributing these products to consumers in the U.S. is prohibited under the Federal Food, Drug, and Cosmetic (FD&C) Act. Failure to promptly correct the violations can result in additional FDA actions such as an injunction, seizure and/or civil money penalties. In addition, products that appear to be misbranded or adulterated that are offered for import into the U.S. are at risk of being detained or refused admission.
Retailers and distributors should communicate with their suppliers to discuss possible options for the unauthorized products in their inventory.
- Wizman Limited doing business as Wizvapor
Puff Boy
Mini Beeper 2.0
Walkie Vape (Pre-Filled Disposable ENDS)
- Shenzhen Fumot Technology Co. Ltd., doing business as R and M Vapes
Fumot Original R and M Max Pro Rechargeable 3600 Puff Disposable Vape Pen Pod Device
R and M Squid Bar 2500 Puffs Disposable Vape Pen Device
R and M Squid Box 5200 Puffs Disposable Vape Pod Device
R and M Switch 2 in 1 Disposable Pod Device (Cartoon Design 2400 Puffs)
Fumot Original R and M Dazzle 5000 RGB Glowing Disposable Vape Pod Device
Fumot Original R and M Dazzle Pro 2600 Puffs RGB Glowing Disposable Vape Pod Device
- Shenzhen Quawins Technology Co., Ltd.
Quawins VFUN Disposable Kit with Rainbow Flash Light
Quawins VFUN PLUS D1 Disposable Kit 3500 Puffs
Quawins VFUN D2 Disposable Kit 1000 Puffs
Quawins Cocktail D1 PV Panda 3000 Puffs Dual Flavor Disposable Kit
- Ruthless Vapor
- Loaded TFN Disposable – Banana Ice
- Loaded TFN Disposable – Mango Banana Ice
- Loaded TFN Disposable – Strawberry Banana Ice
- Moti Global
MOTI POPI Disposable 1500 Puffs Lush Ice
MOTI POPI Disposable 1500 Puffs Juicy Grape
MOTI POPI Disposable 1500 Puffs Strawberry Ice Cream
The FDA closely monitors retailer, manufacturer, importer, and distributor compliance with Federal tobacco laws and regulations and takes corrective action when violations occur. In particular, the agency seeks to enforce where possible against regulated tobacco products that are labeled, advertised, and/or designed to encourage initiation and use of tobacco products by our nation’s youth.
Anyone can report a suspected violation of the Tobacco Control Act or other related regulations, such as selling to underage consumers or selling flavored cigarettes. Unexpected health problems or product problems related to any tobacco product can be reported to FDA through the Safety Reporting Portal.