WARNING LETTER
Wizman Limited d/b/a Wizvapor MARCS-CMS 645185 —
- Delivery Method:
- VIA Electronic Mail
- Product:
- Tobacco
- Recipient:
- Wizman Limited d/b/a Wizvapor
Hong Kong SAR China
-
- info@wizman710.com
- wizmanhk@gmail.com
- marketing@wizman710.com
- Issuing Office:
- Center for Tobacco Products
United States
November 16, 2022
WARNING LETTER
The Center for Tobacco Products of the U.S. Food and Drug Administration (FDA) recently reviewed the website https://www.wizvapor.com and determined that electronic nicotine delivery system (ENDS) products listed there are manufactured and offered for sale or distribution to customers in the United States.
Under section 201(rr) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. § 321(rr)), these products are tobacco products because they are made or derived from tobacco and intended for human consumption. Certain tobacco products, including ENDS, are subject to FDA jurisdiction under section 901(b) of the FD&C Act (21 U.S.C. § 387a(b)) and 21 C.F.R. § 1100.1. Therefore, ENDS are required to be in compliance with the requirements in the FD&C Act.
Please be aware that, effective August 8, 2016, FDA deemed additional products meeting the definition of a tobacco product, except accessories to these newly deemed products, to be subject to regulation under the FD&C Act. These products include, but are not limited to, ENDS (including e-cigarettes and e-liquids), cigars, and pipe tobacco. See Final Rule, Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products, 81 Fed. Reg. 28,974 (May 10, 2016), available at https://federalregister.gov/a/2016-10685.
Generally, to be legally marketed in the United States, the FD&C Act requires “new tobacco products” to have a premarket authorization order in effect. A “new tobacco product” is any tobacco product that was not commercially marketed in the United States as of February 15, 2007, or any modified tobacco product that was commercially marketed after February 15, 2007 (section 910(a) of the FD&C Act; 21 U.S.C. § 387j(a)). Generally, a marketing authorization order under section 910(c)(1)(A)(i) of the FD&C Act (21 U.S.C. § 387j(c)(1)(A)(i)) is required for a new tobacco product unless (1) the manufacturer of the product submitted a report under section 905(j) of the FD&C Act (21 U.S.C. § 387e(j)) and FDA issues an order finding the product substantially equivalent to a predicate tobacco product (section 910(a)(2)(A) of the FD&C Act) or (2) the manufacturer submitted a report under section 905(j)(1)(A)(ii) of the FD&C Act (21 U.S.C. § 387e(j)(1)(A)(ii)) and all modifications are covered by exemptions from the requirements of substantial equivalence granted by FDA under section 905(j)(3) of the FD&C Act (21 U.S.C. § 387e(j)(3)).
New Tobacco Products Without Required Marketing Authorization are Adulterated and Misbranded
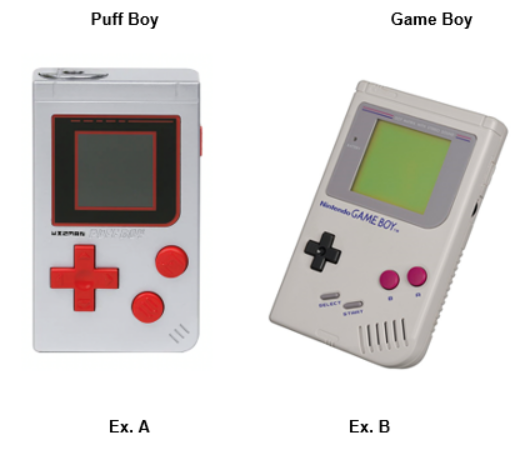
Our review of the website https://www.wizvapor.com revealed that you manufacture and offer for sale or distribution to customers in the United States the following ENDS products without a marketing authorization order, including: Puff Boy; Mini-Beeper 2.0 and Walkie Vape.
The ENDS products listed above are new tobacco products because they were not commercially marketed in the United States as of February 15, 2007. These products do not have an FDA marketing authorization order in effect under section 910(c)(1)(A)(i) of the FD&C Act and are not otherwise exempt from the marketing authorization requirement. Therefore, these products are adulterated under section 902(6)(A) of the FD&C Act. In addition, these products are misbranded under section 903(a)(6) of the FD&C Act because a notice or other information respecting these products were not provided as required by section 905(j) of the FD&C Act.
Additional Considerations
FDA finds your products particularly concerning because the product labeling and/or advertising for Wizvapor Puff Boy, Mini-Beeper 2.0, and Walkie Vape (see Exhibits A) are likely to promote use of the product by youth by imitating children’s toys and gaming products (see Exhibits B). Further, the products’ packaging is likely to promote the use of the products by youth because they help conceal the nature of the product as a tobacco product from parents, teachers, or other adults, and therefore could be openly carried without revealing to parents, teachers, or other adults that the products are tobacco products. FDA is concerned about the rising youth appeal and dramatic rise in youth use of ENDS products. Any efforts to entice youth to use tobacco products are of concern to FDA. Sales of such unauthorized products are prohibited, and FDA is concerned that your actions likely encourage unlawful sales, maintain or increase youth use, and contribute to the public health and safety concerns associated with ENDS products.
Conclusion and Requested Actions
It is your responsibility to ensure that your tobacco products and all related labeling and/or advertising on this website, on any other websites (including e-commerce, social networking, or search engine websites), in any other media in which you advertise, and in any retail establishments comply with each applicable provision of the FD&C Act and FDA’s implementing regulations. Failure to address any violations of the FD&C Act, 21 U.S.C. § 301 et seq., relating to tobacco products including the tobacco regulations in 21 C.F.R. Parts 1140, 1141, and 1143, may result in FDA initiating action, including, but not limited to, civil money penalties, seizure, and/or injunction. However, this Warning Letter does not constitute “written notice” for purposes of section 303(f)(9)(B)(i)(II) of the FD&C Act. Please note that tobacco products offered for import into the United States that appear to be adulterated or misbranded may be subject to detention and refusal of admission.
The violations discussed in this letter do not necessarily constitute an exhaustive list. You should address any violations that are referenced above, as well as violations that are the same as or similar to those stated above, and promptly take any necessary actions to bring your tobacco products into compliance with the FD&C Act.
Please submit a written response to this letter within 15 working days from the date of receipt describing your actions to address any violations and bring your products into compliance, including the dates on which you discontinued the violative labeling, advertising, sale, and/or distribution of these tobacco products and your plan for maintaining compliance with the FD&C Act. If you believe that your products are not in violation of the FD&C Act, include your reasoning and any supporting information for our consideration. This letter notifies you of our findings and provides you with an opportunity to address them. You can find the FD&C Act through links on FDA’s homepage at http://www.fda.gov.
Please note your reference number, RW2201852, in your response and direct your response via email at CTPCompliance@fda.hhs.gov and to the following address:
DPAL-WL Response, Office of Compliance and Enforcement
FDA Center for Tobacco Products
c/o Document Control Center
Building 71, Room G335
10903 New Hampshire Avenue
Silver Spring, MD 20993-0002
If you have any questions about the content of this letter, please contact Bryan Hills at (301) 796-9367 or via email at CTPCompliance@fda.hhs.gov.
Sincerely,
/S/
Ann Simoneau, J.D.
Director
Office of Compliance and Enforcement
Center for Tobacco Products
VIA Electronic Mail
cc:
Withheld for Privacy ehf
b88884b4025b4e8c848e137f814a43e4.protect@withheldforprivacy.com
NameCheap, Inc.
abuse@namecheap.com
Shopify, Inc.
abuse@shopify.com