COMPANY ANNOUNCEMENT
Tenacore LLC Issues Nationwide Recall of Tenacore’s Replacement for the Front Bezel Assembly of the CareFusion Alaris 8100 Infusion Pump Module
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Medical Devices
- Reason for Announcement:
-
Recall Reason DescriptionPotentially weakened plastic
- Company Name:
- Tenacore LLC
- Brand Name:
-
Brand Name(s)Tenacore LLC
- Product Description:
-
Product Description2001 Tenacore Replacement CareFusion Alaris 8100 bezels
Company Announcement
On February 24th, 2021, Tenacore LLC initiated a nationwide recall of 2001 Tenacore replacement CareFusion Alaris 8100 bezels due to potentially weakened plastic. A bezel with weakened plastic may, over time, lead to separation of the bezel post (recall issue) as well as other damage to the bezel (e.g., external cracking). The separation of one or more bezel posts may result in free flow, over infusion, under infusion or interruption of infusion.
There is a related recall initiated by Becton Dickinson (BD). Information regarding that recall can be found using the following link:
Consumers who have:
- Tenacore bezel parts (part number TIPA-8100-4410) with timestamp 5 and/or timestamp 6 should stop the distribution and use of these and return them to Tenacore LLC.
- Alaris 8100 units that were serviced by Tenacore, or purchased from Tenacore between July 2020 and Feb 2021, should be inspected per the instructions described below to ensure that your device is not impacted. If it is, please return your device to Tenacore LLC.
Recalled bezels were manufactured from May 2020 to June 2020 and distributed from July 2020 through Feb 2021.
The following products have been recalled:
|
Name of Product |
UDI |
Model(s) |
Serial Number(s) |
Quantity |
|---|---|---|---|---|
| Bezel for repair of CareFusion Alaris 8100 (TIPA-8100-4410) |
N/A | N/A | Timestamp 5 and Timestamp 6 | 1511 |
| Pre-owned Alaris 8100 units purchased from Tenacore | N/A | N/A | Please contact us for the serial number list | 388 |
| Alaris 8100 units repaired by Tenacore | N/A | N/A | Please contact us for the serial number list | 102 |
Product(s) can be identified by:
If you purchased parts from Tenacore:
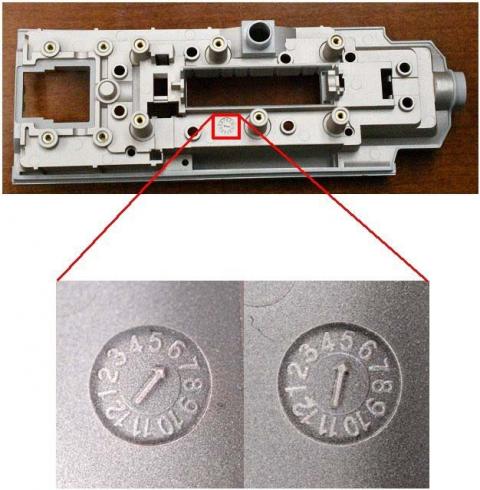
- Look at the rear side of the bezel.
- Find the timestamp as shown in the picture below.
- If the timestamp shows a 5 or 6, please follow the instructions in the notice for returning these impacted parts to Tenacore.
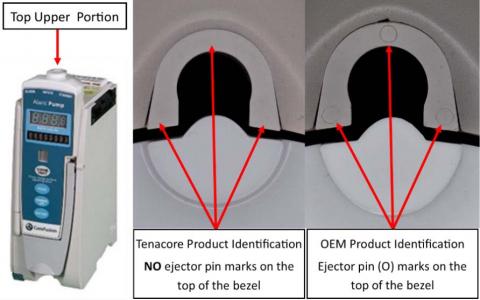
If your Alaris 8100 device was repaired by Tenacore or you purchased it from Tenacore:
- Looking from the top down for the ejector pin marks shown in the image below.
- If NO ejector pin marks are identified, this module requires replacement of the bezel.
Tenacore LLC voluntarily recalled the bezel part for repair of CareFusion Alaris 8100 after becoming aware of possible cracking and separation of the bezel post, as shown below. Tenacore LLC has notified the FDA of this action.
A bezel with weakened plastic may, over time, lead to separation of the bezel post (recall issue) as well as other damage to the bezel (e.g., external cracking). The separation of one or more bezel posts may result in free flow, over infusion, under infusion or interruption of infusion.
Tenacore LLC is notifying its distributors and customers by phone call, email, and physical mail; and is arranging for return of all recalled product in order to replace or credit where applicable.
Tenacore LLC distributed the bezel nationwide.
Consumers with questions may contact the company via telephone at provide 1-800-297-2241 between the hours of 8:00 AM and 4:00 PM (Pacific Time). Consumers may also contact the company via e-mail at quality@tenacore.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.