COMPANY ANNOUNCEMENT
Sciegen Pharmaceuticals, Inc. Issues Voluntary Nationwide Recall of Irbesartan Tablets, USP 75 Mg, 150 Mg, and 300 Mg Due to The Detection of Trace Amounts of NDEA (N-Nitrosodiethylamine) Impurity Found in The Active Pharmaceutical Ingredient (API)
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
Prescription Drugs - Reason for Announcement:
-
Recall Reason DescriptionDue to detection of NDEA (N-Nitrosodiethylamine) Impurity
- Company Name:
- ScieGen Pharmaceuticals, Inc
- Brand Name:
-
Brand Name(s)WP Westminster, more
- Product Description:
-
Product DescriptionIrbesartan Tablets, USP 75 mg, 150 mg, and 300 mg dosage forms
Company Announcement
ScieGen Pharmaceuticals, Inc. is voluntarily recalling listed lots, within expiry, of Irbesartan Tablets, USP 75 mg, 150 mg, and 300 mg dosage forms to the consumer level. These products are being recalled due to the presence of an impurity, N-nitrosodiethylamine (NDEA) contained in the API Irbesartan, USP manufactured by Aurobindo Pharma Limited. This impurity, which is a substance that occurs naturally in certain foods, drinking water, air pollution, and industrial processes, has been classified as a probable human carcinogen as per International Agency for Research on Cancer (IARC)
To date, Sciegen Pharmaceuticals Inc has not received any reports of adverse events related to this product.
Irbesartan tablets, USP are indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.
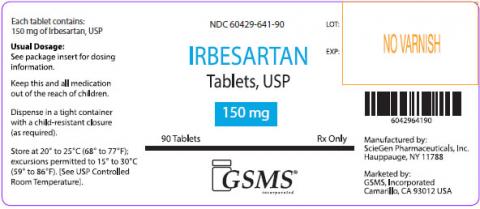
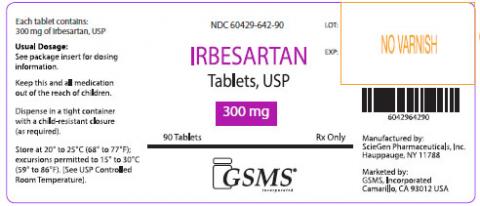
Irbesartan Tablets, USP 75 mg, 150 mg, and 300 mg were manufactured by ScieGen Pharmaceuticals Inc and are labeled as Westminster Pharmaceuticals and Golden State Medical Supply, Inc [GSMS].
The recalls and returns will be managed by the respective distributors separately for the lots distributed by them as outlined below.
Details of batches sent to Westminster
The Irbesartan tablets subject to recall are packed in 30-count and 90-count bottles. To help identify the recalled product, the NDCs, product description, lot numbers and expiration dates are listed below. These lots were distributed nationwide in the USA to Westminster’s direct accounts.
| NDC# | Product Description | Lot# | Expiration Date |
|---|---|---|---|
| 69367-119-01 | Irbesartan 75mg Tablets, 30 count bottle | B160002A | Sep-19 |
| 69367-119-03 | Irbesartan 75mg Tablets, 90 count bottle | B160002B | Sep-19 |
| 69367-120-01 | Irbesartan 150mg Tablets, 30 count bottle | B161005A | Sep-19 |
| C161002A | Feb-20 | ||
| 69367-120-03 | Irbesartan 150mg Tablets, 90 count bottle | B161005B | Sep-19 |
| C161002B | Feb-20 | ||
| 69367-121-01 | Irbesartan 300mg Tablets, 30 count bottle | B162008A | Sep-19 |
| C162002A | Feb-20 | ||
| 69367-121-03 | Irbesartan 300mg Tablets, 90 count bottle | B162008B | Sep-19 |
| C162002B | Feb-20 |
Westminster is notifying its direct accounts by email and by phone to immediately discontinue distribution of the product being recalled and to notify their wholesale and retail accounts of this product recall and make arrangements for impacted product to be returned to Westminster. Instructions for returning recalled products are provided in the Recall Notice Letter and Recall Response Form. Patients should return the effected medication to their pharmacy. Pharmacies should return their effected stock to their wholesaler.
If you are taking Irbesartan, please examine your tablets and look for the specific markings to determine if you’re product is affected by this recall. Products can be best identified by patients as being white, oval shaped tablets debossed with SG 160; SG 161; or SG 162.
Customers and patients with medical-related questions, information about an adverse event or other questions about the Westminster’s product’s being recalled should contact Westminster’s Regulatory Affairs department by phone at: 888-354-9939
- Live calls are received Monday-Friday, 9:00AM - 5:00PM EST with voicemail available 24 hours/day, 7 days/week or email recalls@wprx.com.
Details of batches sent to Golden State Medical Supply, Inc [GSMS]
The products subject to recall are packed in 30-count and 90-count bottles. To help identify the recalled product, the NDCs, Product Description, Lot numbers and Expiration dates are listed below. These lots were distributed nationwide in the USA to GSMS’ direct accounts.
| NDC# | Product Description | Lot# | Expiration Date |
|---|---|---|---|
| 60429-641-30 | Irbesartan 150mg Tablets, 30 Count Bottle | GS019526 | Nov-19 |
| GS020252 | Nov-19 | ||
| GS020958 | Nov-19 | ||
| 60429-642-30 | Irbesartan 300mg Tablets, 30 Count Bottle | GS019036 | Sep-19 |
| GS019073 | Sep-19 | ||
| GS021472 | Nov-19 | ||
| GS021530 | Nov-19 | ||
| GS022234 | Feb-20 | ||
| 60429-640-90 | Irbesartan 75mg Tablets, 90 Count Bottle | B160003 | Sep-19 |
| B160004 | Sep-19 | ||
| 60429-641-90 | Irbesartan 150mg Tablets, 90 Count Bottle | B161003 | Sep-19 |
| B161004 | Sep-19 | ||
| B161006 | Sep-19 | ||
| B161007 | Sep-19 | ||
| B161008 | Nov-19 | ||
| B161009 | Nov-19 | ||
| B161010 | Nov-19 | ||
| C161001 | Feb-20 | ||
| C161003 | May-20 | ||
| 60429-642-90 | Irbesartan 300mg Tablets, 90 Count Bottle | B162009 | Sep-19 |
| B162010 | Sep-19 | ||
| B162011 | Sep-19 | ||
| B162012 | Nov-19 | ||
| B162013 | Nov-19 | ||
| B162014 | Nov-19 | ||
| B162015 | Nov-19 | ||
| C162001 | Feb-20 |
Complete the Recall Inventory Response Form and return to Golden State Medical Supply Incorporated email: recalls@gsms.us or by via Fax: (805) 477-9869 Contact Golden State Medical Supply Incorporated for directions on return authorizations by calling (800) 284-8633 ext. 215 between 7:30AM-4:00PM Pacific; or email: recalls@gsms.us.
If you are taking Irbesartan, please examine your tablets and look for the specific markings to determine if you’re product is affected by this recall. Products can be best identified by patients as being white, oval shaped tablets debossed with SG 160; SG 161; or SG 162.
Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication. Patients who are on Irbesartan should continue taking their medication, as the risk of harm to a patient’s health may be higher if the treatment is stopped immediately without any alternative treatment. Patients should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using Irbesartan.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being made with the knowledge of the Food and Drug Administration.
Company Contact Information
- Consumers:
- Westminster’s Regulatory Affairs, Golden State Medical Supply Incorporated
- 888-354-9939, (800) 284-8633 ext. 215
- Media:
- Siva Reddy P.V
- 1)-855-724-3436