COMPANY ANNOUNCEMENT
Oscor Inc. Issues Recall Product Expansion of TB – Temporary Bipolar Pacing Lead (Unshrouded 2 mm Pins Models)
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionConnector cap housing may slide and potentially expose the connection wire
- Company Name:
- Oscor Inc.
- Brand Name:
-
Brand Name(s)Oscor
- Product Description:
-
Product DescriptionTemporary Bipolar Pacing Lead, Model TB
Company Announcement
On September 26, 2018 Oscor notified customers of a recall for certain lots (Recall No. 1035166- 09/07/2018-01-R) of TB Unshrouded Bipolar Pacing Leads. As part of the recall correction activities, Oscor is retrieving any remaining inventory out in the field. The recall scope is being expanded to include expired inventory for devices distributed between December 21, 2011 to May 17, 2018. The recall expansion is to ensure proper disposition of expired units. The FDA has been notified and is aware Oscor Inc. is voluntarily taking this action.
INTENDED USE:
The Temporary Bipolar Pacing Lead, Model TB is used transvenously for temporary pacing and sensing of the heart in conjunction with a compatible external pulse generator.
DEVICE DESCRIPTION:
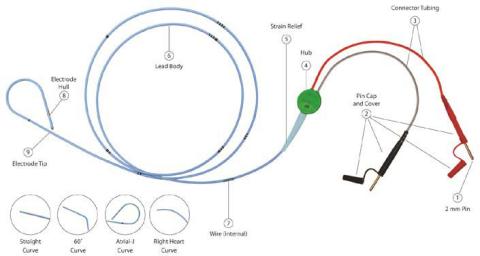
The temporary pacing lead is a bipolar lead with depth markings. The proximal end of the lead includes two 2mm (unshrouded) connectors which fit directly to most pulse generators or extension cable. The French size is printed on the lead hub.
REASON FOR THE VOLUNTARY PRODUCT REMOVAL:
During the use of some TB - Temporary Bipolar Pacing Leads, featuring the 2mm unshrouded connectors, the connector cap housing (see Picture 1, No. 2 Pin Cap and Cover) may slide and potentially expose the connection wire. In some instances, this may cause the wire to be more susceptible to loss of connectivity or breakage during movement of the cables causing interruption of the pacing system. The analysis of the returned devices attributed the failure to a design change of the cap housing of the pins. In the last six years, a total of four serious injuries were reported to Oscor which were attributed to a connector cap malfunction causing the lead connector to separate during use potentially leading to an interruption of the pacing system. No deaths were reported; however the risk for serious injury and/or death is a concern if the connectors separates during use.
EVENT DESCRIPTION: During the use of some TB - Temporary Bipolar Pacing Leads, featuring the 2mm unshrouded connectors, the connector cap housing (see Picture 1, No. 2 Pin Cap and Cover) may slide and potentially expose the connection wire. In some instances, this may cause the wire to be more susceptible to loss of connectivity or breakage during movement of the cables causing interruption of the pacing system.
REASON FOR RECALL: In the last six years, a total of four serious injuries were reported to Oscor which were attributed to the above connector cap malfunction. No deaths were reported; however the risk for serious injury and/or death is a concern if the connectors separates during use.
WARNING:
For pacing dependent patients, an interruption of pacing system could result in serious injury or death if not detected. Continuous monitoring is required.
MODEL NUMBERS:
| GTIN | Model Number | Description | Specifications | |
|---|---|---|---|---|
| French size | Curve Type | |||
| 00836559009726 | 020004 | TB LEAD 4F UN-SHROUDED | 4F | Straight |
| 00836559009733 | 020005 | TB LEAD 5F UN-SHROUDED | 5F | Straight |
| 00836559009740 | 020006 | TB LEAD 6F UN-SHROUDED | 6F | Straight |
| 00836559009788 | 020010 | TB LEAD 4F UN-SHROUDED | 4F | Atrial J |
| 00836559009795 | 020011 | TB LEAD 5F UN-SHROUDED | 5F | Atrial J |
| 00836559009801 | 020012 | TB LEAD 6F UN-SHROUDED | 6F | Atrial J |
| 00836559009856 | 020017 | TB LEAD 5F UN-SHROUDED | 5F | 60° Curve |
| 00836559009863 | 020018 | TB LEAD 6F UN-SHROUDED | 6F | 60° Curve |
| 00836559009900 | 020022 | TB LEAD 4F UN-SHROUDED | 4F | Right Heart |
| 00836559009917 | 020023 | TB LEAD 5F UN-SHROUDED | 5F | Right Heart |
| 00836559009924 | 020024 | TB LEAD 6F UN-SHROUDED | 6F | Right Heart |
| 00836559009030 | TBK04110USG | TB LEAD 4F UN-SHROUDED CONVENIENCE KIT | 4F | Straight |
| 00836559009054 | TBK05110USG | TB LEAD 5F UN-SHROUDED CONVENIENCE KIT | 5F | Straight |
| 00836559009078 | TBK06110USG | TB LEAD 6F UN-SHROUDED CONVENIENCE KIT | 6F | Straight |
| 00885672007027 | TBVK04110USG | TB LEAD 4F UN-SHROUDED CONVENIENCE KIT | 4F | 60° Curve |
| 00885672007034 | TBJK04110USG | TB LEAD 4F UN-SHROUDED CONVENIENCE KIT | 4F | Atrial J |
| 00885672004378 | TBRHK04110USG | TB LEAD 4F UN-SHROUDED CONVENIENCE KIT | 4F | Right Heart |
| 00885672103682 | TBRHK06110USG | TB LEAD 6F UN-SHROUDED CONVENIENCE KIT | 6F | Right Heart |
The Models listed below were not part of the original press release issued by Oscor Inc. on September 24, 2018. |
||||
| 00836559009849 | 020016 | TB LEAD 4F, UNSHOURDED | 4F | 60° Curve |
| 00885672004354 | TBVK06110USG | TB LEAD 6F UN-SHROUDED CONVENIENCE KIT | 6F | 60° Curve |
| 00885672004392 | TBRHK05110USG | TB LEAD 5F UN-SHROUDED CONVENIENCE KIT | 5F | Right Heart |
Customer may contact Oscor’s Customer Relations Group, Monday to Friday from 8:30AM to 5:30PM Eastern Time at 727-937-2511 or via email at TB@oscor.com.
Healthcare professionals are encouraged to report any malfunction and/or adverse events related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
Complete and submit the report Online: www.fda.gov/MedWatch/report.htm
Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.