COMPANY ANNOUNCEMENT
Mylan Institutional LLC Initiates Voluntary Nationwide Recall of Levoleucovorin Injection Due to the Presence of Particulate Matter
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
Generic Drugs - Reason for Announcement:
-
Recall Reason DescriptionPresence of particulate matter
- Company Name:
- Mylan Institutional LLC
- Brand Name:
-
Brand Name(s)Mylan
- Product Description:
-
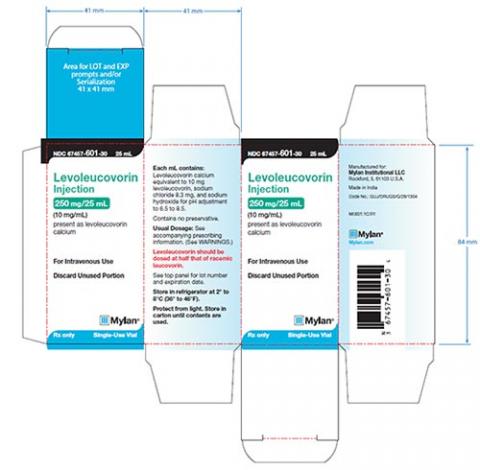
Product DescriptionLevoleucovorin Injection, 250 mg/25 mL
Company Announcement
Mylan Institutional LLC is conducting a voluntary nationwide recall of two lots (see table below) of Levoleucovorin Injection, 250 mg/25 mL to the consumer/user level. The lots were manufactured by Alidac Pharmaceuticals Limited and distributed by Mylan Institutional LLC. The Levoleucovorin Injection is being recalled due to the presence of particulate matter identified as copper salts. The particulate matter was discovered during 12-month stability testing.
Administration of a sterile injectable that has foreign particulates has the potential of severe health consequences. Intravenous administration of a solution containing particulates could lead to local irritation, vasculitis/phlebitis, antigenic or allergic reactions, and microvascular obstruction, including pulmonary embolism. To date, Mylan has not received any reports of adverse events related to this recall.
Levoleucovorin injection is indicated for rescue after high-dose Methotrexate therapy in osteosarcoma; for diminishing the toxicity and counteracting the effects of impaired Methotrexate elimination and of inadvertent overdose of folic acid antagonists; and for the use in combination chemotherapy with 5-fluorouracil in the palliative treatment of patients with advanced metastatic colorectal cancer.
Levoleucovorin Injection, 250 mg contains 25 mL sterile solution in a single-use vial. Each vial is packaged in a carton containing one single-use vial. The batches were distributed in the U.S. between August 2017 and July 2018. The recalled lots are as follows:
| NDC | Product Description and Strength | Size | Lot number | Expiry |
|---|---|---|---|---|
| 67457-601-30 | Levoleucovorin Injection 250 mg/25 mL | 24 mL vial | APB032 | April 2019 |
| 67457-601-30 | Levoleucovorin Injection 250 mg/25 mL | 25 mL vial | APB033 | April 2019 |
Mylan has notified its distributors and customers by letter and is arranging for return of all recalled products. Following are actions for wholesalers, retailers and consumers:
- Wholesaler: Immediately examine your inventory, quarantine and discontinue distribution of these lots. In addition, if you have further distributed the product, please identify your retail level customers and provide a list of customers via Microsoft excel file to mylan7079@stericycle.com within 10 business days. Stericycle will notify your retail level customers that received the affected batches.
- Retailer: Immediately examine your inventory, quarantine and discontinue distribution of these lots. Additionally, if you have further distributed the product, please identify the consumer and notify them immediately of this product recall. The consumer should be instructed to contact Stericycle at 1-866-551-2706 for the documentation packet to return the product.
- Consumer: Please contact Stericycle at 1-866-551-2706 for the documentation packet to return product to Stericycle.
Consumers with questions regarding this recall can contact Mylan Customer Relations at 800.796.9526 or customer.service@mylan.com, Monday through Friday from 8 a.m. – 5 p.m. EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
-
Complete and submit the report Online: www.fda.gov/medwatch/report.htm
-
Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This recall is being executed with the knowledge of the U.S. Food and Drug Administration.