COMPANY ANNOUNCEMENT
Legacy Pharmaceutical Packaging, LLC Issues Voluntary Nationwide Recall of Losartan Potassium Tablets, USP, 25mg, 50mg, and 100mg Due to the Detection of Trace Amounts of N-Nitroso N-Methyl 4-Amino Butyric Acid (NMBA) Impurity Found in the Active Pharmaceutical Ingredient (API)
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
Prescription Drugs - Reason for Announcement:
-
Recall Reason DescriptionDue to The Detection of N-Nitroso N-Methyl 4-Amino Butyric Acid (NMBA)
- Company Name:
- Legacy Pharmaceutical Packaging
- Brand Name:
-
Brand Name(s)Legacy
- Product Description:
-
Product DescriptionLosartan Potassium USP
Company Announcement
Legacy Pharmaceutical Packaging, LLC is recalling 40 repackaged lots of Losartan Tablets USP 25mg, 50mg, and 100mg to the consumer level. This recall was prompted due to Camber Pharmaceuticals, Inc. issuing a Voluntary Nationwide Recall of Losartan Tablets, USP, due to the detection of trace amounts of N-Nitroso N-Methyl 4-amino butyric acid (NMBA) a possible process impurity or contaminant in an active pharmaceutical ingredient, manufactured by Hetero Labs Limited, (API manufacturer).
NMBA is a potential human carcinogen. To date, Legacy has not received any reports of adverse events related to this recall.
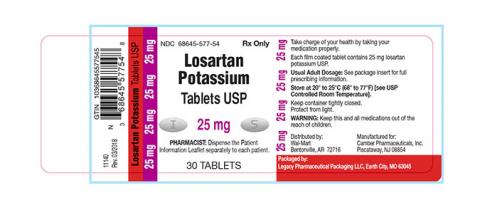
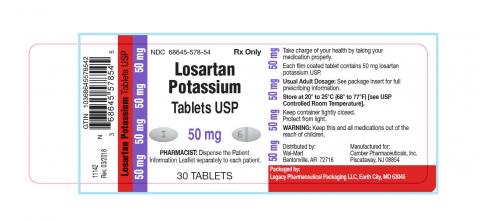
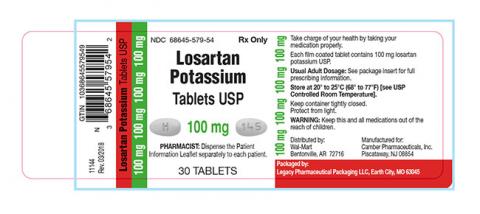
Losartan Potassium USP is a prescription medication used to treat high blood pressure and congestive heart failure and is packaged in 30ct bottles. The identifying NDC numbers associated with Legacy’s product are as follows:
Losartan Potassium, USP, 25mg NDC 68645-577-54
Losartan Potassium, USP, 50mg NDC 68645-578-54
Losartan Potassium, USP, 100mg NDC 68645-579-54
The affected Losartan Potassium includes 40 repackaged lots numbers which are listed below:
| LEGACY NDC# | Name and Strength | Count | Legacy Lot # | Expiry |
|---|---|---|---|---|
| 68645-577-54 | Losartan Potassium Tablets USP 25 mg | 30 | 180952 | 10/2019 |
| 68645-577-54 | Losartan Potassium Tablets USP 25 mg | 30 | 180953 | 12/2019 |
| 68645-577-54 | Losartan Potassium Tablets USP 25 mg | 30 | 181086 | 09/2019 |
| 68645-577-54 | Losartan Potassium Tablets USP 25 mg | 30 | 181572 | 01/2020 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 180921 | 09/2019 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 180922 | 10/2019 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 180923 | 11/2019 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 180924 | 11/2019 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 181118 | 11/2019 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 181119 | 10/2019 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 181407 | 11/2019 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 181408 | 12/2019 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 181573 | 02/2020 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 181725 | 02/2020 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 181726 | 02/2020 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 181948 | 03/2020 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 181960 | 02/2020 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 182385 | 03/2020 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 182386 | 03/2020 |
| 68645-578-54 | Losartan Potassium Tablets USP 50 mg | 30 | 182387 | 03/2020 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 180886 | 11/2019 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 180887 | 12/2019 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 180888 | 12/2019 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 180905 | 12/2019 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181123 | 09/2019 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181124 | 10/2019 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181125 | 08/2019 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181351 | 11/2019 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181352 | 12/2019 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181551 | 11/2019 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181628 | 06/2020 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181629 | 06/2020 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181727 | 06/2020 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181728 | 06/2020 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181890 | 03/2020 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181891 | 06/2020 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 181897 | 06/2020 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 182114 | 03/2020 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 182119 | 06/2020 |
| 68645-579-54 | Losartan Potassium Tablets USP 100 mg | 30 | 182120 | 06/2020 |
The product can be identified by checking the product name and repackaged lot number on the bottle containing these products.
Losartan Potassium was distributed by pharmacies nationwide. Legacy Pharmaceutical Packaging LLC is notifying its distributors and customers in writing and is arranging/assisting for return of all recalled products to (a) For Distribution Center Level, please return the products to Legacy Pharmaceutical Packaging LLC; (b) For Retail Level, please return the products to Genco; or (c) For Consumer Level, please return the products to the dispensing pharmacy, whichever is applicable. Instructions for returning recalled products are provided in the recall letter.
If you have any medical questions regarding this recall, please contact Camber Pharmaceuticals, Inc. at 1-866-495-1995 (8:00 am – 4:30 pm Eastern Time).
Consumers with questions regarding this recall can contact your dispensing pharmacy during normal business hours. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems associated with the use of this product may be reported to FDA's MedWatch Adverse Event Reporting program either by phone, on line, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration
Company Contact Information
- Consumers:
- Dispensing Pharmacy
- 314-813-1555
- Media:
- Legacy Pharmaceutical Packaging