COMPANY ANNOUNCEMENT
International Laboratories, LLC Issues Voluntary Nationwide Recall of one (1) Lot of Clopidogrel Tablets USP, 75 mg packaged in bottles of 30 tablets Due to Mislabeling NDC # 54458-888-16; Lot # 117099A
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read AnnouncementSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
- Company Name:
- International Laboratories, LLC
- Brand Name:
-
Brand Name(s)International Laboratories, LLC
- Product Description:
-
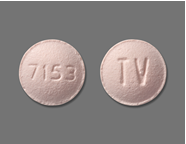
Product DescriptionClopidogrel Tablets USP, 75 mg
Company Announcement
International Laboratories, LLC is voluntarily recalling Lot# 117099A of Clopidogrel Tablets, USP 75 mg, packaged in bottles of 30 tablets, to the consumer level due to mislabeling. The product is labeled as Clopidogrel tablets USP 75 mg but may contain Clopidogrel 75mg or Simvastatin Tablets USP 10 mg.
Missed doses of Clopidogrel increases the risk of heart attack and stroke which can be life threatening. Patients should not stop taking clopidogrel without talking to their prescribing physician. Additionally, unintentional consumption of simvastatin could include the common side effects associated with its use and may cause fetal harm when administered to a pregnant woman. Simvastatin occasionally causes myopathy which is a disease of the muscles. Finally, allergic reactions are also possible and could also be life threatening. International Laboratories, LLC also reports that to date, no complaints have been received related to this event detailing medical illnesses or harmful effects.
Clopidogrel Tablets USP 75 mg are a platelet inhibitor (blood thinner) indicated for the use in patient with acute coronary syndrome, recent MI, recent stroke, or established peripheral arterial disease. Clopidogrel tablets have been shown to reduce the rate of MI and stroke.
The product was distributed nationwide and delivered to the distribution centers in Arkansas, Georgia, Indiana, California and Maryland, and distributed to retail stores in all US States.
International Laboratories, LLC is notifying distributors and customers by letter and is arranging for return of all recalled products. Consumers who have purchased this product should stop using and return the product to the location of purchase for a full refund. For questions regarding return of product please call Inmar at 855-258-7280 or via email internationallabs@inmar.com or by using mailing address Recall Coordinator 635 Vine St. Winston Salem, NC 27101 Inmar’s business hours are (Monday – Friday 9 AM – 5 PM EST).
Consumers should also contact their physician or healthcare provider if they are experiencing any health concerns that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.