COMPANY ANNOUNCEMENT
Incredible Products Sa De Cv Issues Voluntary Nationwide Recall of Gelbac T Antibacterial Handgel
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionMethanol Contamination
- Company Name:
- Incredible Products Sa de Cv
- Brand Name:
-
Brand Name(s)Gelbac T
- Product Description:
-
Product DescriptionAntibacterial Handgel
Company Announcement
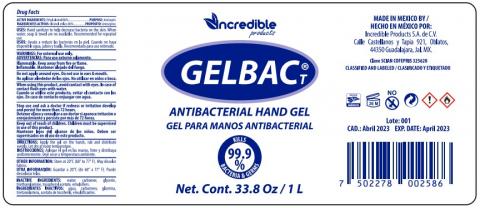
INCREDIBLE PRODUCTS SA DE CV is voluntarily recalling 1 lot of GELBAC T ANTIBACTERIAL HANDGEL, packaged in 1 liter /33.8 oz plastic bottles and 125ml/4.2 oz plastic bottles of to the consumer level. The products are being recalled due to the potential presence of methanol (wood alcohol). FDA sampling of product at the port of entry to contain undeclared methanol.
Risk Statement: Substantial methanol exposure could result in nausea, vomiting, headache, blurred vision, permanent blindness, seizures, coma, and permanent damage to the nervous system or death. Although all persons using these products on their hands are at risk, young children who accidentally ingest these products and adolescents and adults who drink these products as an alcohol (ethanol) substitute are most at risk for methanol poisoning. To date, INCREDIBLE PRODUCTS SA DE CV has not received reports of adverse events related to this recall.
These products are used as hand sanitizers and marketed to help decrease bacteria on the skin when soap and water are not available. The affected Hand Sanitizers are packaged in clear plastic bottles. The recalled products are as follows:
Product, Sizes and LOT:
GELBAC T ANTIBACTERIAL HANDGEL
Sizes 33.8 oz, 4.2 oz
LOT CODE 001
INCREDIBLE PRODUCTS SA DE CV is notifying its distributors and customers by recall letter and consumers via this press release and is arranging for refund of all recalled products.
Consumers with questions regarding this recall can contact INCREDIBLE PRODUCTS SA DE CV by sending an email to gcigdl@yahoo.com.mx and dalp_07@hotmail.com during business hours.
Contact
Jesus Guillermo Vazquez Ramirez
FDA updates on hand sanitizers consumers should not use.