COMPANY ANNOUNCEMENT
Fresenius Kabi Issues Voluntary Nationwide Recall of Two Lots of Fluorouracil Injection Due to the Potential for Glass Particulate
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
Generic Drugs - Reason for Announcement:
-
Recall Reason DescriptionPotential for glass particulate
- Company Name:
- Fresenius Kabi USA, LLC
- Brand Name:
-
Brand Name(s)Fresenius Kabi, Novaplus
- Product Description:
-
Product DescriptionFluorouracil Injection
Company Announcement
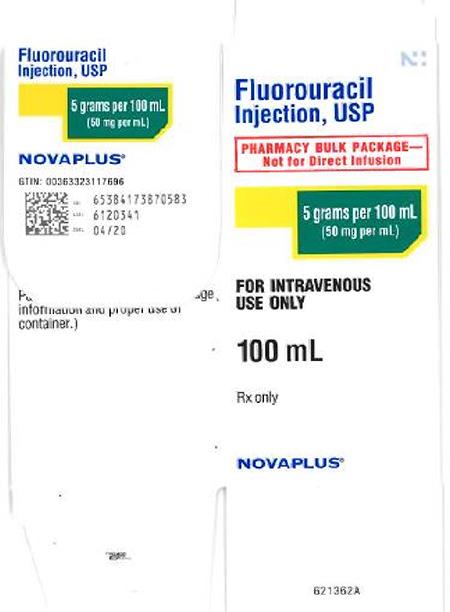
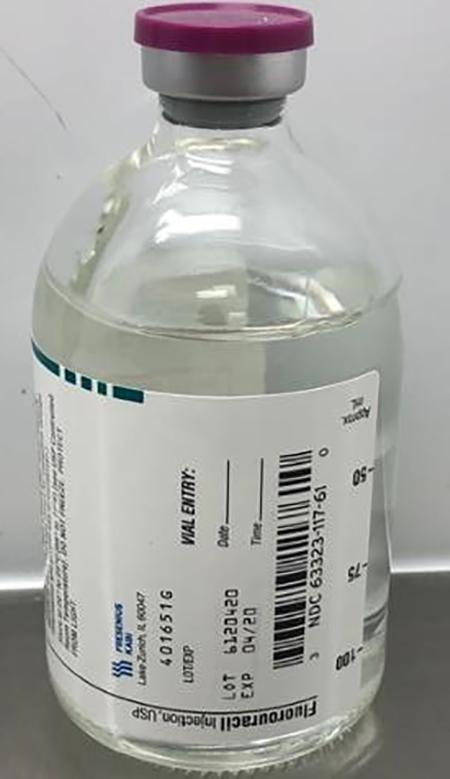
Fresenius Kabi USA, LLC is voluntarily recalling two lots of Fluorouracil Injection, USP 5g/100mL (50mg/mL), 100mL fill in 100mL vials, to the user level due to the potential for glass particulate. The affected lots, distributed between December 6, 2018 and February 20, 2019, are listed below:
| Product Name/Size | NDC Number |
Product Code |
Lot Number |
Expiration Date |
First Ship Date |
Last Ship Date |
|---|---|---|---|---|---|---|
| Fluorouracil Injection, USP, 5g/100mL (50mg/mL), 100mL fill in a 100mL vial |
63323-117-69 | NP101761 | 6120341 | 04-2020 | 12/06/2018 | 12/18/2018 |
| 63323-117-61 |
101761 |
6120420 | 04-2020 | 12/07/2018 | 02/20/2019 |
Products containing glass particulate should not be administered intravenously due to the potential for life-threatening consequences. Reports in the literature suggest that sequelae of thromboembolism, such as pulmonary emboli, phlebitis, granulomas, or fibrosis may occur.
To date, Fresenius Kabi has not received any complaints or reports of adverse events related to this recall.
The company is issuing this notification after finding glass particulate in five vials in retained sample inventory of lot 6120341 during an inspection for a quality investigation. The second lot (6120420) is included in the recall as a precautionary measure as it was produced in the same filling campaign.
Fluorouracil is a chemotherapy drug that is administered intravenously and indicated for the treatment of a variety of cancers.
Fresenius Kabi is notifying its distributors and customers by letter and asking customers and distributors to check their stock immediately and to quarantine and discontinue the use and distribution of any affected product. Distributors should notify their customers and direct them to quarantine and discontinue distributing or dispensing any affected lots, and to return the product to Fresenius Kabi. The recall letter and response form are available at https://www.fresenius-kabi.com/us/pharmaceutical-product-updates.
Consumers with questions regarding this recall may contact Fresenius Kabi at 1-800-551-7176 Monday through Friday, during the hours of 8:00 a.m. to 5:00 p.m. or via email at productcomplaint.USA@fresenius-kabi.com or adverse.events.USA@freseniuskabi.com Consumers should contact their physician or health care provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. For more information about Fresenius Kabi worldwide, please visit www.fresenius-kabi.com.
Company Contact Information
- Consumers:
- Fresenius Kabi
- 1-800-551-7176
- productcomplaint.USA@fresenius-kabi.com, adverse.events.USA@fresenius-kabi.com
- Media:
- Matt Kuhn
- (847) 550-5751
- matt.kuhn@fresenius-kabi.com