COMPANY ANNOUNCEMENT
Fresenius Kabi Issues Voluntary Nationwide Recall of 13 Lots of Ketorolac Tromethamine Injection, USP Due to the Presence of Particulate Matter in Reserve Samples
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionPresence of Particulate Matter
- Company Name:
- Fresenius Kabi USA, LLC
- Brand Name:
-
Brand Name(s)Fresenius Kabi
- Product Description:
-
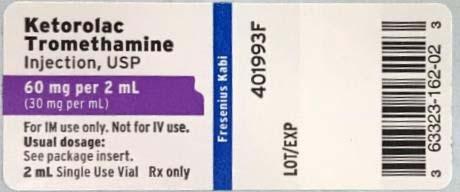
Product DescriptionKetorolac Tromethamine Injection, USP, 30 mg/mL, and Ketorolac Tromethamine Injection, USP, 60 mg/2 mL
Company Announcement
Fresenius Kabi USA, LLC is voluntarily recalling 13 lots of Ketorolac Tromethamine Injection, USP, 30 mg/mL, 1 mL fill in a 2 mL amber vial and Ketorolac Tromethamine Injection, USP, 60 mg/2 mL (30 mg/mL), 2 mL fill in a 2 mL amber vial to the user level due to the presence of particulate matter composed of the following elements: carbon, silicon, oxygen and polyamides. Particulate matter was found in eight reserve sample vials.
Administration of products containing particulate matter could obstruct blood vessels and result in local irritation of blood vessels, swelling at the site of injection, a mass of tissue that could become inflamed and infected, blood clots traveling to the lung, scarring of the lung tissues, and allergic reactions that could lead to life-threatening consequences.
Ketorolac Tromethamine, a nonsteroidal anti-inflammatory drug, is indicated for the shortterm (up to 5 days in adults) management of moderately severe acute pain that requires analgesia at the opioid level. The total combined duration of use of oral Ketorolac Tromethamine and Ketorolac Tromethamine injection should not exceed 5 days.
Listed below is a table of the recalled lots distributed nationwide to wholesalers, distributors, hospitals, and pharmacies between May 5, 2018 and December 16, 2019, as well as a copy of the label:
|
Product Name/Product size |
NDC Number |
Product Code |
Batch Number |
Expiration Date |
First Ship Date |
Last Ship Date |
|---|---|---|---|---|---|---|
| Ketorolac Tromethamine Injection, USP, 30 mg / mL, 1 mL fill in a 2 mL amber vial |
63323-162-01 |
160201 |
6118737 | 04/2020 | 05/30/2018 | 06/27/2018 |
| 6118902 | 04/2020 | 08/01/2018 | 08/15/2018 | |||
| 6119052 | 05/2020 | 06/25/2018 | 07/25/2018 | |||
| 6119752 | 08/2020 | 09/28/2018 | 12/06/2018 | |||
| 6122349 | 07/2021 | 09/16/2019 | 11/04/2019 | |||
| 6122538 | 09/2021 | 11/01/2019 | 12/16/2019 | |||
| Ketorolac Tromethamine Injection, USP, 60 mg / 2 mL (30 mg / mL), 2 mL fill in a 2 mL amber vial | 63323-162-02 | 160202 | 6119229 | 06/2020 | 08/09/2018 | 10/30/2018 |
| 6119273 | 06/2020 | 09/26/2018 | 03/30/2019 | |||
| 6119843 | 09/2020 | 11/11/2019 | 01/07/2020 | |||
| 6121115 | 02/2021 | 03/30/2019 | 04/22/2019 | |||
| 6121451 | 03/2021 | 04/29/2019 | 08/05/2019 | |||
| 6121452 | 03/2021 | 07/12/2019 | 10/22/2019 | |||
| 6121496 | 03/2021 | 06/21/2019 | 12/10/2019 |
Fresenius Kabi is notifying its distributors and customers by letter and asking customers and distributors to check their stock immediately and to quarantine and discontinue the use and distribution of any affected product. Distributors should notify their customers and direct them to quarantine and discontinue distributing or dispensing any affected lots, and to return the product to Fresenius Kabi. The recall letter and response form are available at https://www.fresenius-kabi.com/us/pharmaceutical-product-updates.
Customers with questions regarding this recall may contact Fresenius Kabi at 1-866-716-2459 Monday through Friday, during the hours of 8:00 a.m. to 5:00 p.m. Central Time. Consumers should contact their physician or health care provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
- Or, contact Fresenius Kabi at 1-800-551-7176, Monday through Friday, during the hours of 8:00 a.m. to 5:00 p.m. or via email at: productcomplaint.USA@fresenius-kabi.com or adverse.events.USA@fresenius-kabi.com
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
About Fresenius Kabi
Fresenius Kabi (www.fresenius-kabi.com/us) is a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. For more information about Fresenius Kabi worldwide, please visit www.fresenius-kabi.com
Company Contact Information
- Consumers:
- Fresenius Kabi
- 1-866-716-2459
- Media:
- Matt Kuhn
- 847- 550-5751
- matt.kuhn@fresenius-kabi.com