COMPANY ANNOUNCEMENT
Exela Pharma Sciences, LLC Issues Voluntary Nationwide Recall of Ibuprofen Lysine Injection, 20 Mg/2 Ml (10 Mg/Ml) Due to Particulate Matter
This recall has been completed and FDA has terminated this recall.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
Generic Drugs - Reason for Announcement:
-
Recall Reason DescriptionSome of the vials have been found to contain particulate matter
- Company Name:
- Exela Pharma Sciences, LLC
- Brand Name:
-
Brand Name(s)X-Gen
- Product Description:
-
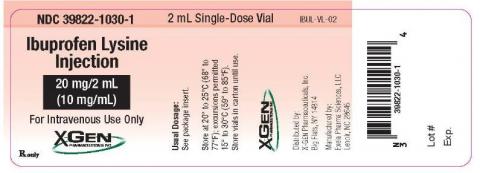
Product DescriptionIbuprofen Lysine Injection, 20 mg/2 mL
Company Announcement
FOR IMMEDIATE RELEASE – 02/08/2017 – Lenoir, NC. Exela Pharma Sciences, LLC (“Exela”), in association with marketer X-Gen Pharmaceuticals, Inc. (“X-Gen”), is voluntarily recalling lot number PLND1613 of Ibuprofen Lysine Injection, 20 mg /2 mL (10 mg/mL), vials to the hospital or user level. Some of the vials have been found to contain particulate matter.
Risk Statement: Particulate matter has the potential to block blood vessels, provoke an immune reaction, and/or lead to microinfarcts which could be life threatening. Neither Exela nor X-Gen has received any reports of adverse events related to this recall.
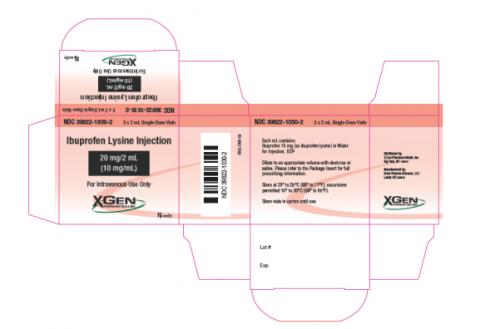
Ibuprofen Lysine Injection is indicated to close a clinically significant patent ductus arteriosus (PDA) in premature infants weighing between 500 and 1500 g, who are no more than 32 weeks gestational age when usual medical management is ineffective. The product is packaged in three 2 mL Single-Dose vials per carton, and bears the NDC 39822-1030-2. The affected Ibuprofen Lysine Injection, 20 mg/2 mL (10 mg/mL) is from lot PLND1613, Expiration Date 02/2018. The product can be identified by X-Gen logo, and by the NDC number on the individual vial (39822-1030-1). The product was distributed nationwide to wholesalers and distributors for further distribution to hospitals and retail customers.
X-Gen is notifying its distributors and customers by emails and fax communications and is arranging for return of all recalled products. Consumers/distributors/retailers that have the Ibuprofen Lysine Injection which is being recalled should stop using and return to their wholesaler/distributor, or to X-Gen or to Exela.
Consumers with questions regarding this recall can contact Exela at 1-888-451-4231, Monday-Friday between 8 AM and 5 PM EST, or by email at the address shown above; or, they can contact X-Gen at 1-866-390-4411, Monday-Friday between 8 AM and 5 PM EST, or by email at Recall@x-gen.us. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- Exela, X-Gen
- 1-888-451-4231, 1-866-390-4411
- Recall@x-gen.us
- Media:
- Lisabeth Wiles
- 828-758-5474 x115
- lwiles@exela.us