GUIDANCE DOCUMENT
Uniform Contraceptive Labeling - Guidance for Industry July 1998
- Docket Number:
- FDA-2020-D-0957
- Issued by:
-
Guidance Issuing OfficeCenter for Devices and Radiological Health
Document issued on: July 23, 1998
U.S. Department Of Health and Human Services
Food and Drug Administration
Center for Devices and Radiological Health
Obstetrics and Gynecology Devices Branch
Division of Reproductive, Abdominal, Ear, Nose and Throat,
and Radiological Devices
Office of Device Evaluation
Preface
Public Comment
Comments and suggestions may be submitted at any time for Agency consideration to Colin Pollard, HFZ-470, Obstetrics and Gynecology Devices Branch, Office of Device Evaluation, FDA, 9200 Corporate Boulevard, Rockville, Maryland, 20850. Comments may not be acted upon by the Agency until the document is next revised or updated. For questions regarding the use or interpretation of this guidance contact Colin Pollard at 240-276-4155 or by e-mail at colin.pollard@fda.hhs.gov.
Additional Copies
Additional copies are available from the Internet. You may also send an e-mail request to CDRH-Guidance@fda.hhs.gov to receive a copy of the guidance. Please use the document number 1251 to identify the guidance you are requesting.
Uniform Labeling for Contraceptive Devices: Guidance to Industry
Background
This guidance provides agency recommendations for the labeling of all contraceptive devices regarding protection from pregnancy and sexually transmitted diseases. Much of this information has already been transmitted over the years to the industry in a variety of letters and guidance documents. This guidance document is an effort to bring all of these previous recommendations together into one document that will serve to encourage a uniform approach to this type of labeling information for the consumer. Where appropriate, this same information should be included in the professional labeling for these devices. It should be noted that these recommendations are the result of an intra-agency effort that involved CDRH’s Office of Device Evaluation and Office of Health and Industry Programs (OHIP), as well as the Division of Reproductive and Urologic Drug Products and the Division of Over-the-Counter Drug Products in the Center for Drug Evaluation and Research. The effort was overseen by FDA’s Office of Women’s Health, which also provided funding for focus studies conducted by OHIP.
Protection from Pregnancy
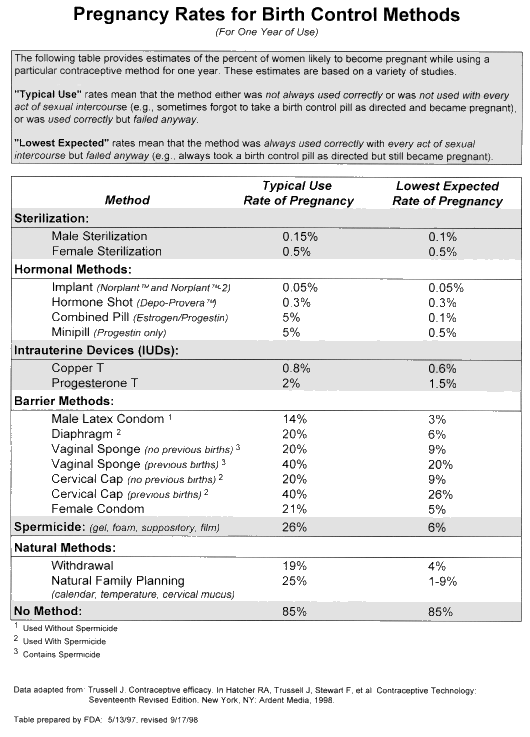
Labeling for all contraceptive devices (including male & female condoms, diaphragms, cervical caps, intrauterine devices (IUDs), and tubal occlusion devices (TODs) should contain an easy-to-read table with the pregnancy rates associated with all methods. This table is intended to provide the device user with pregnancy rate on his/her selected contraceptive, as well as comparative information on the pregnancy rates of various other contraceptive products. The table recommended by FDA uses pregnancy rates based on data from Trussell, et al, from the 17th edition of Contraceptive Technology (1997). Using information from a series of focus studies conducted by the CDRH, FDA developed a re-formatted table (copy attached) that contains the appropriate information in an understandable form to help people appreciate the effectiveness of their contraceptive of choice with respect to the many other products available on the market.
FDA considers the communication of information on pregnancy rates to users of contraceptive devices to be essential for their safe and effective use. Therefore, all contraceptive devices should have labeling with a table like the one recommended in this guidance document. In addition, for those products requiring professional labeling, the same table should be included in an appropriate section of that labeling.
Protection from Sexually Transmitted Diseases (STDS)
In addition to inclusion of a contraceptive efficacy table in the labeling, manufacturers should include information to the user on the ability of that contraceptive device to provide protection from STDs. FDA has requested that manufacturers, depending on the type of contraceptive device, include one of the following statements in their patient/consumer labeling regarding transmission of STDs.
For intrauterine devices (IUDs), tubal occlusion devices, and natural skin condoms:
This product is intended to prevent pregnancy. It does not protect against HIV infection and othersexually transmitted diseases.
For natural skin condoms, the following additional statement should be included:
In order to help reduce the risk of transmission of many STDs, including HIV infections (AIDS), use a latex condom.
For latex condoms for men:
(On the condom wrapper/Principal display panel) If used properly, latex condoms will help to reduce the risk of transmission of HIV infection (AIDS) and many sexually transmitted diseases.
(Directions For Use) If used properly, latex condoms will help to reduce the risk of transmission of HIV infection (AIDS) and many sexually transmitted diseases, including chlamydia, genital herpes, genital warts, gonorrhea, hepatitis B, and syphilis.
For male condoms made from new materials:
This interim labeling is based on slippage & breakage data while a contraceptive effectiveness study is underway.
You may use this [insert name] condom if you or your partner are allergic to latex.
You should know:
- The risks of pregnancy and sexually transmitted diseases (STDs), including AIDS (HIV infection), are not known for this condom. A study is being done.
- There are laboratory tests on this [blank] material. These tests show that organisms even as small as sperm and viruses like HIV cannot pass through it.
Latex condoms [boldface] for men, if used correctly with every act of vaginal intercourse, are highly effective at preventing pregnancy, as well as STDs, including AIDS (HIV infection).
For female condoms, the following key elements are placed on the primary package as well as the package insert:
- Latex condoms for men are highly effective at preventing sexually transmitted diseases, including AIDS (HIV infection), if used properly.
- If you are not going to use a male latex condom, you can use [insert name] to help protect yourself and your partner.
- [Insert name] only works when you use it. Use it every time you have sex.
- Before you try [insert name], be sure to read the directions in the box and learn how to use it properly.
For diaphragms and cervical caps:
FDA is aware of a few clinical studies of diaphragms and vaginal spermicides that look at protection from a few STDs. However, these studies are not conclusive, and no definitive statement that can be used for uniform labeling has been developed. Therefore, no claims for STD protection afforded by diaphragms or cervical caps may be made without submission of an appropriate 510(k) premarket notification or PMA supplement.
References
- Memorandum to FDA’s Acting Director, Office of Women’s Health, May 27, 1997, on Uniform Contraceptive Effectiveness Labeling - Completion of Project.
- Silberberg, Paula, September 1996, Report On Four Focus Groups Among Women On Their Reactions To Two Contraceptive Efficacy Table For Uniform Labeling, Office of Health and Industry Programs, Center for Devices and Radiological Health.
- Hatcher et al., Contraceptive Technology, 17th ed., 1997, Irvington Publishers, Inc.
Submit Comments
You can submit online or written comments on any guidance at any time (see 21 CFR 10.115(g)(5))
If unable to submit comments online, please mail written comments to:
Dockets Management
Food and Drug Administration
5630 Fishers Lane, Rm 1061
Rockville, MD 20852
All written comments should be identified with this document's docket number: FDA-2020-D-0957.