Testimony | In Person
Event Title
Addressing New Variants: A Federal Perspective On The Covid-19 Response
January 11, 2022
- Testimony of
-
Janet Woodcock, M.D.
- Before the
Introduction
Chair Murray, Ranking Member Burr, distinguished members of the Committee, I am Dr. Janet Woodcock, Acting Commissioner of the U.S. Food and Drug Administration (FDA or the Agency). Thank you for the opportunity to testify before you today to describe FDA’s coronavirus disease 2019 (COVID-19) response efforts. All of our efforts are in close coordination and collaboration with our partners, both within the Department of Health and Human Services (HHS) and across the Federal government, to help ensure the development, authorization, licensure, approval, and availability of critical, safe, and effective medical products to address the COVID-19 public health emergency.
I want to note that this testimony is just a snapshot of some of our extensive work and is in the context of efforts across the Agency to address this pandemic. There are thousands of FDA employees who have been working on COVID-19 response efforts non-stop since the start of the pandemic. I want to commend and recognize their efforts and thank them for their dedication and service. I also want to thank all FDA employees who have continued to work on the myriad issues the Agency is responsible for that do not directly involve COVID-19.
From the beginning of this public health emergency, FDA has taken an active leadership role in the all-of-government response to the COVID-19 pandemic, inspired by the resiliency of the American people and our great innovators. FDA stood up an internal cross-agency group that continues to ensure we are doing everything possible to protect the American public, help ensure the safety, efficacy, and quality of FDA-regulated medical products, and provide the industries we regulate with the guidance and tools to do the same. We continue to focus on facilitating the development and availability of medical countermeasures to diagnose, treat, and prevent COVID-19, surveilling the medical product and food supply chains for potential shortages or disruptions, and helping to mitigate such impacts, as necessary to protect the public health.
This includes working to quickly address any potential impacts of the new omicron variant. FDA is working as quickly as possible to evaluate the potential impact of this variant on the currently available diagnostics, therapeutics and vaccines. We are closely monitoring the situation and are committed to communicating with the public as we learn more. Just a couple weeks ago we updated the SARS-CoV-2 Viral Mutations: Impact on COVID-19 Tests web page to share new information on the omicron variant and its impact on antigen diagnostic tests. FDA is committed to continuing to use every tool in our toolbox to fight this pandemic, including pivoting as the virus adapts, to arm ourselves with the best available diagnostics, and life-saving therapeutics and vaccines to fight this virus.
At this time, the current vaccines remain highly effective at preventing serious clinical outcomes associated with a COVID-19 infection, including hospitalization and death. Additionally, currently available data from our international partners and vaccine manufacturers that has been evaluated by the Agency, suggests that an additional booster shot following the completion of a primary vaccination provides further protection.
Getting vaccinated or receiving a booster with one of the currently available vaccines is the best thing Americans can do right now, in addition to standard precautions like wearing a mask, to help protect themselves and their families.
Biologics, Including Vaccines
FDA’s Center for Biologics Evaluation and Research (CBER) continues to use every tool available to help facilitate the development and availability of vaccines and other biological products to combat the COVID-19 pandemic expeditiously and safely.
CBER is working on multiple fronts to address the COVID-19 pandemic, including:
- Helping to facilitate expedited clinical trials for vaccines and certain therapeutic biological products that hold promise to prevent or treat COVID-19 by providing timely interactions, scientific advice, and recommendations for individual sponsors and through issuance of guidance documents;
- Supporting product development and facilitating the scaling up of manufacturing capacity for high priority products to treat COVID-19 and conducting timely reviews;
- Expediting the review of Emergency Use Authorization (EUA) requests and Biologics License Applications (BLAs) for vaccines and other critical medical products to address COVID-19, including the evaluation of booster doses of COVID-19 vaccines and the use of COVID-19 vaccines in certain pediatric populations;
- Helping to ensure an adequate and safe blood supply; and
- Providing information to healthcare providers and researchers to help them submit expanded access investigational new drug application (IND) requests to permit the use of CBER-regulated investigational products for patients with COVID-19.
CBER’s work on COVID-19 vaccines, as discussed below, has made a tremendous difference in addressing the pandemic by facilitating the availability of COVID-19 vaccines, that meet the Agency’s rigorous standards, as expeditiously as possible. Through our transparent scientific evaluation process, FDA has issued EUAs for three COVID-19 vaccines and has approved one vaccine for use in individuals 16 years of age and older. In doing so, we have relied upon the Agency’s rigorous standards for safety, effectiveness, and manufacturing quality. These COVID-19 vaccines were developed without cutting corners or compromising our regulatory and scientific standards. Intensive interactions between FDA and manufacturers minimized the time between different studies in the clinical development process; allowed seamless movement throughout the different phases of clinical trials; and simultaneously facilitated manufacturers proceeding with manufacturing scale-up before it was clear whether the safety and effectiveness data for a vaccine would support an EUA, allowing for quicker access to products once FDA reviewed the data and found the products met the Agency’s rigorous standards for authorization or approval.
For the approved vaccine, as well as those that have been authorized for emergency use, our process included a thorough evaluation of the data by the Agency’s career staff. We also solicited input from independent scientific and public health experts through our public advisory committee meetings for the COVID-19 vaccines that we have authorized. Throughout our scientific and regulatory process, FDA took additional steps to facilitate transparency, such as posting sponsor and FDA briefing documents and key decisional memoranda.
The COVID-19 vaccines that are available in the United States have shown clear and compelling efficacy in large, well-designed phase 3 trials. These vaccines are helping the country in the fight against this pandemic and have met FDA’s rigorous standards for safety and effectiveness to support either EUA or approval. All the COVID-19 vaccines that FDA has authorized for emergency use have far surpassed being at least 50 percent more effective than a placebo in preventing COVID-19, which was recommended in our June 2020 guidance document, Development and Licensure of Vaccines to Prevent COVID-19.1 A vaccine with at least 50 percent efficacy, we noted, would have a significant impact on disease, both at the individual and societal level. The vaccines are approved or authorized to prevent COVID-19, and have been shown to significantly reduce the associated serious outcomes, including hospitalization and death.
During this past year, we have continued to make great strides with regard to COVID-19 vaccines. As part of our continued efforts to be transparent and educate the public, we have a wealth of information on our website about the COVID-19 vaccines available for use in the United States. The information includes fact sheets for healthcare providers (vaccination providers) and fact sheets for vaccine recipients and caregivers in multiple languages, with important information such as dosing instructions; information about the benefits and risks of each vaccine; and topical Questions and Answers developed by FDA for the approved vaccine and each authorized vaccine.2
It is also important to highlight that, as part of each EUA or approval, manufacturers and vaccination providers are required to report serious adverse events, cases of Multisystem Inflammatory Syndrome (MIS), and cases of COVID-19 that result in hospitalization or death to the Vaccine Adverse Event Reporting System (VAERS), a national vaccine safety surveillance program jointly run by FDA and the Centers for Disease Control and Prevention (CDC).
COVID-19 vaccine safety is a top priority for the federal government, and we take all reports of health problems following COVID-19 vaccination very seriously. FDA and CDC have implemented a coordinated and overlapping approach for continuous safety monitoring of all COVID-19 vaccines using state-of the art technologies. Specifically, the Agency’s monitoring following authorization of the COVID-19 vaccines uses a multi-pronged approach including: 1) passive surveillance using VAERS consisting of safety reports submitted by healthcare providers (providers in the CDC COVID-19 Vaccination Program are required to report adverse events following COVID-19 vaccination to VAERS), patients, parents and other members of the public, combined with 2) active surveillance, using large population-based healthcare datasets. These latter healthcare data systems offer a higher likelihood of detecting rare adverse events because they capture medical data on millions of Americans, cover diverse subpopulations (i.e., pregnant women, elderly, and patients with comorbidities) and can provide a longer duration of follow-up when compared to the prelicensure clinical studies. In addition, COVID-19 vaccine recipients are encouraged to enroll in CDC’s v-safe After Vaccination Health Checker smartphone-based tool that uses text messaging and web surveys to check-in with vaccine recipients over time after they receive a COVID-19 vaccine. Through v-safe, they can quickly tell CDC if they have any side effects after getting a COVID-19 vaccine. Together, the passive and active safety surveillance provide a coordinated and overlapping approach to vaccine safety monitoring for COVID-19 vaccines.
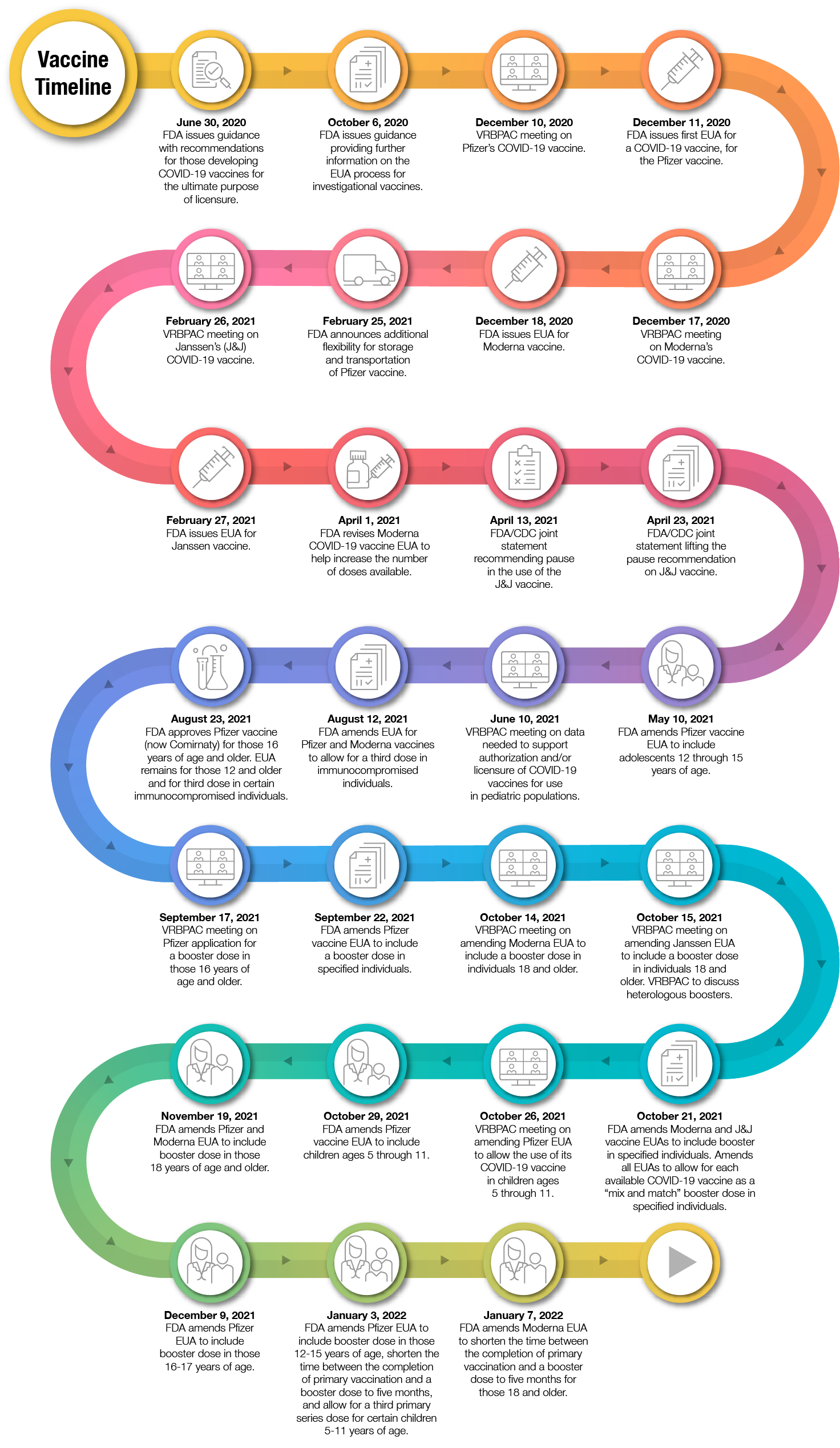
On August 23, 2021, FDA announced the first approval of a COVID-19 vaccine. The vaccine previously known as the Pfizer-BioNTech COVID-19 Vaccine was approved and is now marketed as Comirnaty, for the prevention of COVID-19 in individuals 16 years of age and older. Comirnaty has the same formulation as the originally authorized Pfizer-BioNTech COVID-19 Vaccine. Since the approval of Comirnaty, the Pfizer-BioNTech COVID-19 Vaccine has continued to be available under an EUA, including for the two-dose primary series in individuals 12 through 15 years of age and as a third primary series dose for individuals 5 years of age and older who have been determined to have certain kinds of immunocompromised conditions. While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. To be clear, the American public should feel confident in receiving any of the available vaccines.
On September 22, 2021, FDA amended the EUA for the Pfizer-BioNTech COVID-19 Vaccine to allow for use of a single booster dose, to be administered at least six months after completion of the primary series in the following groups: individuals 65 years of age and older, individuals 18 through 64 years of age at high risk of severe COVID-19, and individuals 18 through 64 years of age whose frequent institutional or occupational exposure puts them at high risk of serious complications of COVID-19 including severe COVID-19.
On October 20, 2021, FDA further amended the EUA to clarify that a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine may also be administered at least 6 months after completion of the primary series to individuals 18 through 64 years of age with frequent institutional or occupational exposure to severe acute respiratory syndrome coronavirus 2(SARS-CoV-2).
On October 20, 2021, FDA also amended the Moderna COVID-19 Vaccine EUA to include use of a single booster dose at least 6 months after completion of the primary series in the following groups: individuals 65 years of age and older, and those 18-64 years of age at high-risk of severe COVID-19 or with frequent institutional or occupational exposure to SARS-CoV2. The Agency also amended the Janssen EUA to include the use of a single booster dose of the Janssen (Johnson & Johnson) COVID-19 Vaccine, administered at least 2 months after completion of the single-dose primary regimen to individuals 18 years of age and older. As of this announcement, all three COVID-19 vaccines had been authorized for a booster dose, but with varying eligibility.
Additionally, FDA authorized the use of heterologous, or “mix and match,” booster dosing in eligible individuals following completion of primary vaccination with a different available COVID-19 vaccine.
On October 29, 2021, the FDA authorized the emergency use of the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include children 5 through 11 years of age. The authorization was based on the FDA’s thorough and transparent evaluation of the data that included input from independent advisory committee experts who overwhelmingly voted in favor of making the vaccine available to children in this age group. We are confident in the safety, effectiveness and manufacturing data behind this authorization. As part of our commitment to transparency around our decision-making, which included a public advisory committee meeting, we have posted documents supporting our decision. We hope this information gives parents the confidence they need to have their children vaccinated.
On the same day, FDA also authorized a manufacturing change for the vaccine to include a formulation that uses a different buffer; buffers help maintain a vaccine’s pH (a measure of how acidic or alkaline a solution is) and stability. This authorization is for two presentations: one for individuals 12 years of age and older and one for individuals 5 through 11 years of age. This new formulation is more stable at refrigerated temperatures for longer periods of time, permitting greater flexibility for vaccination providers. The new formulation of the vaccine developed by Pfizer Inc. contains Tris buffer, a commonly used buffer in a variety of other FDA-approved vaccines and other biologics, including products for use in children. FDA evaluated manufacturing data to support the use of Pfizer-BioNTech COVID-19 Vaccine containing Tris buffer and concluded it does not present safety or effectiveness concerns. FDA has since approved this new Tris formulation as part of the Comirnaty BLA.
On November 19, 2021, FDA amended the EUA for both the Moderna and Pfizer-BioNTech COVID-19 vaccines authorizing use of a single booster dose for all individuals 18 years of age and older six months after completion of primary vaccination with any FDA-authorized or approved COVID-19 vaccine. On December 9, 2021, FDA amended the EUA for the Pfizer-BioNTech COVID-19 Vaccine, authorizing the use of a single booster dose for administration to individuals 16 and 17 years of age at least six months after completion of primary vaccination with the Pfizer-BioNTech COVID-19 Vaccine.
On January 3, 2022, FDA amended the EUA for the Pfizer-BioNTech COVID-19 Vaccine to expand the use of a single booster dose to include use in all individuals 12 through 15 years of age after completion of primary vaccination and authorized a third primary series dose for certain immunocompromised children 5 through 11 years of age. Additionally, FDA authorized the shortening of the time between the completion of primary vaccination of the Pfizer-BioNTech COVID-19 Vaccine and a booster dose to at least five months. Last Friday, January 7, 2022, FDA also amended the EUA for the Moderna COVID-19 Vaccine to shorten the time between the completion of a primary series of the vaccine and a booster dose to at least five months for individuals 18 years of age and older.
At this time FDA is closely monitoring the emergence of the Omicron variant in order to determine what, if anything, needs to be changed in the composition of COVID-19 vaccines moving forward to best protect the population. The Agency has already issued COVID-19 vaccine-specific guidance to address the emergence and potential future emergence of variants of SARS-CoV-2, the virus that causes COVID-19.
Figure 1
This pandemic is dynamic and evolving, with new data continuously emerging about vaccine safety and effectiveness. As we obtain more data about the safety and effectiveness of COVID-19 vaccines, including the use of a booster dose, we will continue to evaluate the rapidly changing science and keep the public informed.
At this time, it is clear that the approved or authorized vaccines reduce the risk of severe illness; however, data are not yet available to make a determination about how long they will provide protection. Additionally, although we do not yet know the full range of SARS-CoV-2 variants that each of the vaccines will protect against, there is evidence that the available vaccines protect against disease caused by variants circulating in the United States.
Finally, manufacturers whose COVID-19 vaccines have been authorized for emergency use are expected to continue their clinical trials in order to obtain additional safety and effectiveness information and pursue licensure (approval).
To date, having three authorized vaccines and one approved vaccine that meet FDA’s expectations for safety and effectiveness at this point of the COVID-19 pandemic is a tremendous achievement and a testament to the dedication of vaccine developers and FDA’s career scientists and physicians. We are highly engaged in ensuring that all COVID-19 vaccines meet the high quality that the American public expects and deserves. The Agency is very proud of these efforts, and we believe that the vaccines will help bring this pandemic to an end.
In addition to its work on COVID-19 vaccines, CBER also has been actively involved in reviewing data related to COVID-19 convalescent plasma and on December 28, 2021, the FDA updated the EUA for COVID-19 convalescent plasma. The update limits the authorization to the use of COVID-19 convalescent plasma with high titers of anti-SARS-CoV-2 antibodies for the treatment of COVID-19 in patients with immunosuppressive disease or who are receiving immunosuppressive treatment. These patients may be treated in outpatient or inpatient settings. Additionally, to help assure the manufacture of high titer COVID-19 convalescent plasma, the update to the EUA revises acceptable tests and increases qualifying result cutoffs to be used for manufacturing COVID-19 convalescent plasma with high titers of anti-SARS-CoV-2 antibodies.
Drug Products
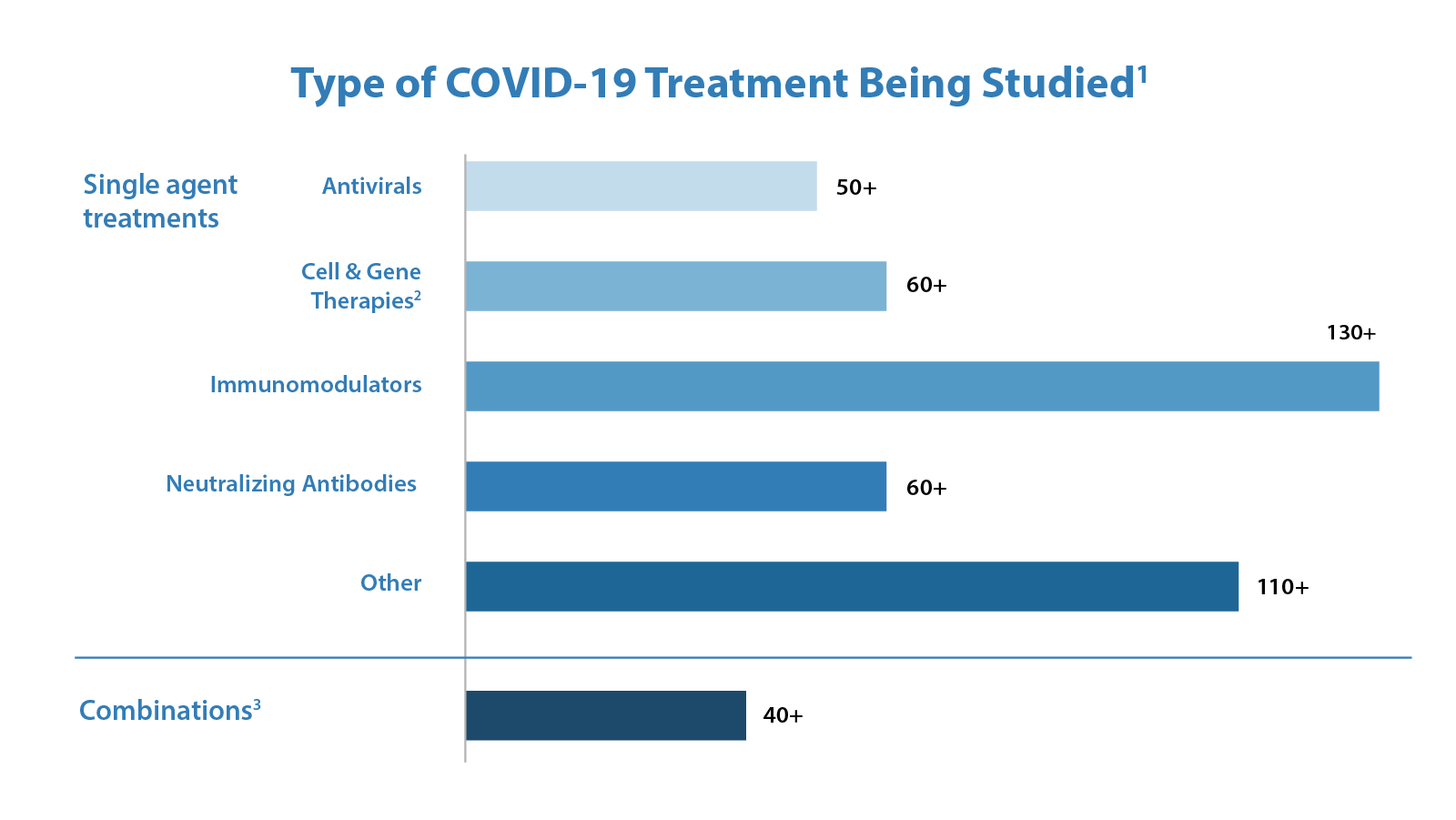
Since the beginning of the COVID-19 pandemic, FDA’s Center for Drug Evaluation and Research (CDER) has been working tirelessly to facilitate the development and availability of therapeutics for use by patients, physicians, and health systems as expeditiously and safely as possible. FDA accelerated the development and publication of guidance and other information for industry and researchers on developing COVID-19-related treatments. Further, on March 31, 2020, FDA announced the creation of an emergency review and development program for possible therapies for COVID-19, the Coronavirus Treatment Acceleration Program, or “CTAP.” The primary goal of CTAP is to help accelerate the development of therapeutics for patients and consumers. The Agency has supported the program by reassigning staff and working continuously to review requests from companies and researchers who are working to develop therapies. Under CTAP, FDA is using every available authority and regulatory flexibility to facilitate the development of safe and effective products to treat patients with COVID-19. As of November 30, 2021, there are more than 670 drug development programs in the planning stages and the Agency has reviewed more than 470 trials of potential therapies for COVID-19. These include antivirals, immunomodulators, neutralizing antibodies, cell and gene therapies, and combinations of these products. The diversity of therapeutic approaches being investigated is important because it rapidly expands our understanding of the effect of different categories of potential treatments.
Figures 2 & 3
1 Corresponds to number of safe to proceed INDs. Excludes INDs related to vaccines
2 For additional information, please see Cellular & Gene Therapy Products
3 Includes INDs with more than one product
As of December 31, 2021, FDA has approved one drug to treat COVID-19 and fourteen therapeutics are currently authorized for emergency use. On December 8, 2021, FDA issued an EUA for AstraZeneca’s Evusheld (tixagevimab co-packaged with cilgavimab and administered together) for the pre-exposure prophylaxis (prevention) of COVID-19 in certain adults and pediatric individuals (12 years of age and older weighing at least 40 kilograms [about 88 pounds]).
On December 22, 2021 FDA issued an EUA for the first oral antiviral, Paxlovid, manufactured by Pfizer. Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) is authorized for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kilograms or about 88 pounds) with positive results of direct SARS-CoV-2 testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death
On December 23, 2021, FDA issued an EUA for another oral antiviral, molnupiravir, manufactured by Merck. Molnupiravir is authorized for the treatment of mild-to-moderate coronavirus disease (COVID-19) in adults with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death, and for whom alternative COVID-19 treatment options authorized by the FDA are not accessible or clinically appropriate.
Both Paxlovid and Molnupiravir are available by prescription only.
In considering EUA requests for therapeutics, we promptly and carefully evaluate the totality of the scientific evidence to determine whether the statutory criteria for issuance under section 564 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3) are met. Among other criteria, this evaluation considers whether the product may be effective for its proposed authorized uses, and whether the product’s known and potential benefits outweigh its risks.
Our goal is to be as transparent as possible about the scientific basis for recommending that a drug or biological product be authorized for emergency use under section 564 of the FD&C Act or for recommending that an EUA be revised or revoked. For example, last month, FDA held a meeting of its Antimicrobial Drugs Advisory Committee (AMDAC) to discuss Merck and Ridgeback’s request for an EUA for molnupiravir, an investigational antiviral drug to treat COVID-19. The advisory committee discussed the available data supporting the use of molnupiravir to treat mild-to-moderate COVID-19 in adults who have tested positive for COVID-19, and who are at high risk for progression to severe COVID-19, including hospitalization or death. The meeting was scheduled as soon as possible following the submission of the EUA request by the company. FDA thoroughly evaluated the data and information submitted in the EUA request before the meeting and engaged in a robust public discussion with the advisory committee members.
FDA continues to work closely with manufacturers to mitigate and prevent shortages as the COVID-19 pandemic evolves. For example, the Agency has issued four EUAs to authorize the emergency use of certain therapeutic products intended to treat serious or life-threatening diseases or conditions (e.g., Acute Kidney Injury, Acute Respiratory Distress Syndrome) caused by COVID-19 after determining that sufficient FDA-approved alternatives to these products were not available to fully meet the emergency need. This has helped to alleviate shortages of some therapies that are essential for the care of critically ill COVID-19 patients. FDA is working with manufacturers to increase supplies to meet current demand by expediting review of applications. In addition, the Agency has prioritized the review of generic drug applications for potential treatments and supportive therapies for patients with COVID-19, such as sedatives used in ventilated patients, anticoagulants, and pulmonary medications. In June 2021, FDA reached a milestone of approving 1,000 original and supplemental generic drug applications since the start of the pandemic to help in the treatment of patients with COVID-19. This supports FDA’s everyday mission of improving access to safe, effective, high-quality treatment options, especially during the COVID-19 pandemic.
Medical Devices
FDA’s Center for Devices and Radiological Health’s work to support access to medical devices for the COVID-19 pandemic began in January 2020 - before the Public Health Emergency (PHE) was declared in the U.S. and two months before the pandemic was declared worldwide - due to the immediate need for COVID-19 tests and testing supplies, collection kits, personal protective equipment (PPE), and other devices. The need for medical devices to respond to the COVID-19 pandemic has far exceeded what we experienced in any prior PHE. The first EUAs issued for the COVID-19 PHE were for medical devices, and the volume of EUA requests quickly surpassed (by two orders of magnitude) that of any prior PHE or other situation. Since January 2020, FDA has received over 7,500 EUA requests and Pre EUA (PEUA) submissions for devices. Further, the emergency use requests included submissions for devices that FDA’s Center for Devices and Radiological Health (CDRH) had never received EUA requests for during prior PHEs. This included ventilators and novel devices such as extracorporeal blood purification devices, as well as novel indications for devices such as continuous renal replacement therapy devices. Since the start of the pandemic, FDA has issued EUAs or granted marketing authorization to nearly 2,000 medical devices for COVID-19-related uses. In addition, FDA rigorously monitors safety signals and medical device reports, using the information to publish 23 letters to healthcare providers and 9 safety communications. FDA completed other pivotal work activities such as addressing supply chain shortages and counterfeit products related to COVID-19.
Diagnostic tests are the first line of defense in an outbreak, and FDA plays an important role to ensure these work through the EUA review. The EUA process expedites access to appropriately accurate diagnostic tests during emergencies, when information gaps and false results may adversely affect individual patient care and public health decision making. Through this process, molecular diagnostic tests are able to be developed, validated, authorized, and deployed within weeks rather than several months to over a year, as is typical for test development and traditional premarket submissions. The Agency employed its EUA authorities to facilitate availability of tests in six previous emergencies. Careful review of tests is critical because false test results can adversely impact the nation’s response. In PHEs, FDA is generally open to receiving and reviewing EUA requests for tests from any developer, including commercial kit manufacturers and laboratories, for tests that address the public health need.
FDA sought to facilitate COVID-19 test evaluation and authorization through the development and availability of templates for EUA requests. The templates provide recommendations for test validation and a fill-in-the-blank form to streamline the paperwork and make it easier for developers to provide information in support of a request for an EUA. Since providing the first template in January 2020, FDA has been in daily contact with test developers to answer questions and help them through the EUA process. This has proved to be a helpful tool for many. FDA had as many as ten posted templates and continues to update, add, combine, and remove templates as the science evolves and as necessary to support developers of COVID-19 tests. As of October 8, 2021, these templates have received over 556,635 hits from those visiting FDA’s website. FDA also supported test developers through establishment of a dedicated mailbox, 24-7 toll-free hotline that ran until July 2020, the posting of over 100 frequently asked questions on our website, and by hosting 74 weekly virtual town halls for test developers. The Agency has worked with over 1,000 test developers since January 2020.
The Agency prioritizes review of EUA requests for at-home rapid antigen tests and is actively engaging with test developers to increase their availability. The Agency first discussed this prioritization in the Spring of 2020, during one of its weekly virtual Town Halls on COVID-19 tests, due to their potential impact on test accessibility and public health. To further encourage such test development, on July 29, 2020, FDA posted a template for at-home diagnostic tests. This template includes recommendations for validating over-the-counter (OTC) tests for screening asymptomatic individuals with general performance expectations that are lower than for lab-based tests. The Agency recognizes the benefits of increased availability of OTC tests, and these recommendations have helped to increase OTC screening test availability, particularly rapid antigen tests.
Throughout the pandemic, FDA has also monitored evolving circumstances and growing scientific knowledge and made adjustments when appropriate to help streamline and expedite the path to market for these and other tests as much as possible while assuring they are supported by sound science. In March 2021, FDA obtained results from an NIH-sponsored study that supported further streamlining of FDA’s at-home test recommendations. Based on these data, on March 16, 2021, FDA issued an EUA that provides a streamlined path to authorize tests with at least 80% sensitivity in symptomatic individuals, with sensitivity falling in a range as low as 70% in certain circumstances, for developers to offer their test for OTC serial screening without additional data collection. Multiple tests were authorized under this approach within weeks.
FDA authorized the first at-home test on November 17, 2020. At-home tests, also referred to as self-tests, are those that can be performed by a lay user at home, or in other settings, with a self-collected sample. In Fall 2021, the Agency added home tests from ACON Laboratories, Celltrion Diatrust, iHealth, and InBios International to the growing list of home tests authorized in the U.S. Two of the FDA’s recent authorizations alone may result in up to 400 million more at-home tests available monthly to American consumers by early 2022, based on projections by the manufacturers and dependent on federal subsidies. As of December 31, 2021, FDA has authorized 15 distinct at-home COVID-19 tests.
FDA further streamlined the process for manufacturers developing over-the-counter at-home tests on October 25, 2021, by facilitating at-home single-use testing for symptomatic individuals for tests currently authorized only for serial testing. Developers of certain tests may request authorization to add single-use testing for symptomatic individuals without submitting additional data. For example, right now when people go to a pharmacy to buy an at-home test, they are sold in two-packs. This change would allow tests authorized for single use to be sold in singles, meaning more individual tests for sale potentially at a lower price.
On November 15, 2021, FDA published an update to its Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency that describes our review priorities based on the current needs of the pandemic. Going forward the FDA generally intends to focus its review on EUA requests for the following types of tests:
- At-home and point-of-care (POC) diagnostic tests for use with or without a prescription and that can be manufactured in high volumes;
- Certain high-volume, lab-based molecular diagnostic tests (and home collection kits for use with such tests) that expand testing capacity or accessibility such as through pooling of specimens to increase throughput, testing specimens collected at home and shipped to the lab, screening asymptomatic individuals or detecting multiple different respiratory viruses at once;
- Certain lab-based and POC high volume antibody tests that can measure the amount of antibodies (fully quantitative antibody tests) or the amount of neutralizing antibodies; and
- Tests for which the request is from, or supported by, a U.S. government stakeholder, such as the Biomedical Advanced Research and Development Authority or the National Institutes of Health’s Rapid Acceleration of Diagnostics (RADx) initiative.
These priorities help developers focus their prospective efforts where they are most needed, and reduce inefficient use of developer and FDA time on tests with less public health impact. Ultimately, we anticipate we will receive EUA requests only for those tests identified in the guidance for which the public health need is greatest, and we will be able to focus our attention on the review of such tests.
FDA also worked with NIH to establish an Independent Test Assessment Program (ITAP) to streamline validation and authorization of antigen tests with potential for large-scale manufacturing. This program is an extension of the RADx program which has already supported development of several authorized tests, including the first at-home OTC COVID-19 test. ITAP goes further to conduct studies on over-the-counter tests and work with companies to help them provide complete, high quality submissions for FDA review. ITAP is conducting independent laboratory and clinical evaluations using protocols developed jointly with FDA. FDA uses information from these evaluations in deciding whether to grant EUAs. On December 24, FDA authorized the first at home COVID-19 test where validation data were gathered through ITAP and the second followed shortly after on December 29. We expect to continue shorter review times for such EUA requests due to our partnership with ITAP in establishing the evaluation program to address our regulatory needs.
Going forward, FDA continues to take steps to increase access to reliable, accurate rapid antigen tests. This includes continuing to prioritize review of EUA requests for at-home antigen tests, and increasing staffing on the antigen test review team as resources permit. FDA is actively working to increase the pipeline of at-home tests by engaging with companies to obtain data that can be used to support their EUA, working with developers with authorized POC tests to expand their authorization for at-home use, continuing support of ITAP and engagement with RADx and international regulators, and conducting targeted outreach to manufacturers of home tests in non-U.S. markets.
Because of these various efforts, as of December 31, 2021, FDA has authorized over 400 tests and sample collection devices for SARS-CoV-2. As noted in Figure 4 below, these include 290 molecular tests and sample collection devices, 87 antibody and other immune response tests, and 43 antigen tests. Among these are 16 authorizations for diagnostic tests that can be run at home without a prescription (three molecular and 13 antigen authorizations). We have also authorized 33 tests for serial screening programs (24 antigen and nine molecular). The volume and variety of authorized tests is a testament to FDA’s support of innovative test design and our commitment to public health. FDA will continue to adapt to address public health needs and increase access to tests for consumers, including at-home diagnostic tests, adopting an approach that is grounded in sound science.
Figure 4
In addition to these efforts, FDA has been actively monitoring for the possible emergence of SARS-CoV-2 variants since early in the pandemic and has worked with test developers when a new variant (or mutation) emerges that could impact test performance. FDA also works with test developers, who are required to monitor their authorized test for the impact of viral mutations. As the FDA’s or the developer’s analysis identifies tests whose performance could be impacted by SARS-CoV-2 viral mutations, these tests are added to FDA’s SARS-CoV-2 Viral Mutations: Impact on COVID-19 Tests webpage. This includes posting the latest information on the omicron variant and testing implications as they become available. FDA also works with other agencies and divisions in HHS, such as NIH, as we monitor tests for potential effects of genetic variation on test performance on an ongoing basis.
Since early 2020, FDA has adopted agile, interactive, and innovative approaches to review EUA requests for all types of devices. For example, FDA developed the umbrella EUA approach to efficiently authorize multiple devices of the same type falling within the scope of authorization and meeting the statutory criteria for issuance. The Agency has also issued 28 guidance documents (including 21 revisions) outlining policies to help expand the availability of medical devices needed in response to COVID-19. For example, developers of certain tests offered their tests, upon validation and notification to FDA, prior to issuance of an EUA during Agency review of the EUA request. Further, FDA made several improvements to our EUA review processes to make the most efficient use of our resources, including a front-end triage process to identify devices that would have the greatest impact on the public health. These improvements incorporate the latest information on device availability and shortages, prioritizing novel or critical devices not yet available on the market or those that would address significant device shortages.
Given the magnitude of the COVID-19 PHE, the FDA recognizes that continued flexibility, while still providing necessary oversight, will be appropriate to facilitate an orderly and transparent transition back to the eventual resumption of normal operations. In December 2021, FDA issued draft guidance for public comment to help manufacturers begin to plan a future return to normal operations, including a proposed phased-in transition period and recommendations relating to submitting marketing submissions. The Agency hopes that providing additional transparency on our current thinking now will facilitate advance planning for an orderly transition to normal operations after the public health emergency with fewer supply disruptions for device manufacturers, healthcare providers, and patients.
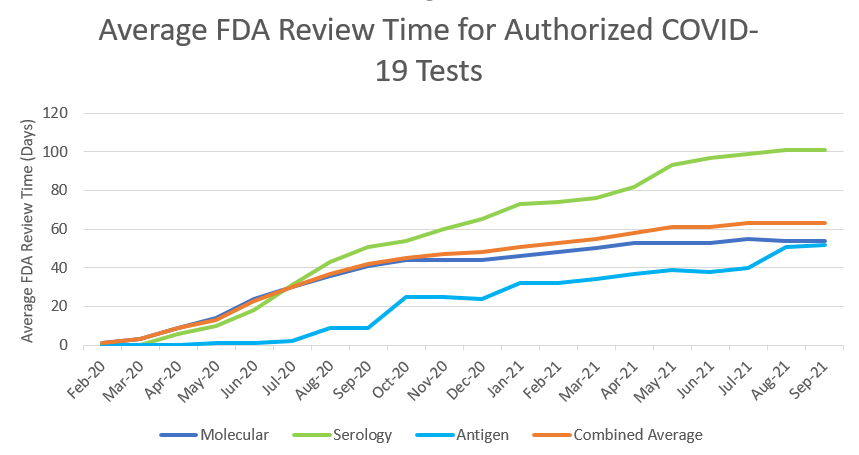
At the beginning of the pandemic, when there were relatively few diagnostic tests authorized, FDA’s priority was to rapidly increase the availability of tests. For medical devices, review times have increased over time as the number of EUA requests and Pre-Emergency Use Authorization (PEUA) submissions for medical devices have increased to unprecedented levels. This is demonstrated in the tables we have provided with review times for IVD EUA requests over time, and submission volume for IVD EUA requests over time (see Figures 5 & 6 below). At the beginning of the pandemic, FDA was authorizing tests and other devices in as little as 1 or 2 days upon receipt of complete data packages. Congress has provided critical, one-time funding that FDA has used to leverage contractors from outside organizations, to provide technical expertise to supplement our review staff in the review of EUA requests and other marketing submissions. These personnel are authorized to work alongside full-time employees, integrated into our internal review teams to help with the massive workload for tests, ventilators, PPE, and other devices, but the workload has continued to greatly exceed capacity even with the additional support.
Figure 5
Figure 6
Please note that FDA’s actions have reduced the review times for tests from a peak average of 90 days for EUA requests received in September 2020 to approximately 35 days for those received since February 2021.
FDA has authorized a wide variety of other medical devices for use in combating the pandemic, including a wide range of PPE, ventilators, and other therapeutic devices. As of December 31, 2021, FDA has authorized 269 PPE devices, including 51 surgical masks, 205 filtering facepiece respirators (FFRs), and issued 13 EUAs for face shields and other barriers intended to protect the user from bodily fluids, liquid splashes, or potentially infectious materials. See Figure 7. In addition to issuing EUAs, FDA has also cleared, through its premarket notification pathway, over 500 PPE 510(k)s, which not only support the response to this pandemic but also future PHEs as well.
Figure 7
FDA recognizes that medical devices, particularly tests, will continue to play an important role in the next phase of the pandemic response. The Agency is continuing to monitor its policies, the marketplace, and national needs, and will continue to adapt as the circumstances of the evolving pandemic warrant.
Human and Animal Food (Center for Food Safety and Applied Nutrition and Center for Veterinary Medicine)
Throughout the pandemic, FDA has worked with Federal, state, and local partners, as well as industry, to help ensure a safe and adequate food supply for both people and animals.
While there is no evidence to show that SARS-CoV-2 is likely to be transmitted by food, some components of the food system are experiencing challenges and supply chain imbalances. We saw this at the outset of the pandemic with the dramatic shift in where people were eating, and most recently, we are seeing that the broad supply chain issues impacting so many commodities are also impacting food. Overall, food production and manufacturing in the U.S. has been remarkably resilient, but we continue to monitor the food supply chain systems closely to efficiently and promptly identify supply chain challenges and apply mitigation strategies when necessary.
In response to the pandemic, FDA’s Foods Program developed 21 Forward, a food supply chain data management tool, to help identify where risks for interruptions in the continuity of the food supply may be greatest. As part of 21 Forward, FDA conducted targeted outreach to the food industry to offer additional resources and technical assistance in addressing challenges.
FDA also recognizes that food supply chain continuity and worker safety are two sides of the same coin. Thus, a robust food supply depends on the safety and health of the nation’s food and agricultural workforce. Along with our Federal, state, and local partners, we have provided best practices for food and agricultural workers, industry, and consumers on how to stay safe, and help ensure the continuity of operations in the food and agriculture critical infrastructure sector during the pandemic.
In collaboration with HHS, CDC, Health Resources and Services Administration (HRSA) and U.S. Department of Agriculture (USDA), data from 21 Forward on the estimated numbers and distribution of food and agricultural workers have been made available to assist states with their vaccine distribution efforts for workers in the food and agriculture sectors, including migratory and seasonal agricultural workers. Now, FDA is using the information from 21 Forward to help identify and react to supply chain challenges and elevate as needed to the appropriate agencies or other entities. In addition, FDA has worked with its Federal partners to provide both COVID-19 and flu vaccination encouragement messages for the food industry.
FDA’s Coordinated Outbreak Response and Evaluation team has been working throughout the pandemic looking for signs of foodborne illness outbreaks and initiating responses as needed. FDA’s Center for Veterinary Medicine is monitoring the animal food supply and initiating needed foodborne illness and natural disaster responses. In terms of inspectional work, FDA’s Office of Regulatory Affairs (ORA) investigators continue to conduct mission-critical inspections domestically and abroad, including inspections and investigations in response to foodborne outbreaks, as they have done throughout the pandemic. FDA resumed standard operations for domestic surveillance inspections in July 2021. FDA continues to screen every line of every shipment of imported food entering the U.S. utilizing our Predictive Risk-Based Evaluation for Dynamic Import Compliance Targeting (PREDICT) tool. We adjusted the algorithm in PREDICT to place increased scrutiny on shipments from facilities where foreign surveillance inspections have been postponed. FDA has made greater use of our Foreign Supplier Verification Program (FSVP) regulation to oversee compliance with FDA Food Safety Modernization Act (FSMA) requirements. The shift to remote FSVP inspections, along with other tools utilized by the foods program, has been critical to ensuring the safety of human and animal food from foreign suppliers during the COVID-19 pandemic. Since March 2020, FDA has conducted approximately 2,982 FSVP inspections, which represents a 95% increase in inspections (1,527) over the 18 months prior to the pandemic. Additionally, FDA continues to identify human and animal foods that are unsafe, misbranded, or may cause a serious health concern for the public at the border with over 10,481 lines being refused admission since March 2020.
In July 2020, FDA announced the New Era of Smarter Food Safety Blueprint outlining the Agency’s plans over the next decade to create a more digital, traceable, and safer food system. The Agency has learned from its response to the pandemic that there is an accelerated need for certain goals in this blueprint, especially those involving supply chain continuity and resilience, modernized inspectional approaches, strengthening food safety infrastructures with regulatory partners, and the safety of foods ordered by consumers online. The number of consumers ordering food online has been steadily increasing over the years, but it has skyrocketed during the COVID-19 pandemic. FDA recently hosted a virtual Summit on E-Commerce to help the Agency improve its understanding of how human and animal foods are sold through e-commerce models and to identify courses of action for addressing potential food safety vulnerabilities, including those that may arise in the “last mile” of delivery.
Inspections, Compliance, and Protecting the Medical Supply Chain
Similar to their work protecting the food supply, import investigators have been on-site protecting the medical supply chain at our ports of entry, courier facilities, and the international mail facilities (IMFs) throughout the pandemic with uninterrupted support from ORA’s laboratories. Through continued vigilance, FDA has prevented unsafe and unproven pharmaceuticals and other medical products from entering the country. Since March 2020, with the cooperation of and in coordination with the U.S. Customs and Border Protection (CBP), FDA has received and destroyed almost 85,500 products, totaling over 15,050,242 capsules, tablets, and other dosage forms of unapproved drugs.
Since March 2020, FDA has maintained the same level of screening for imported products as pre-pandemic and refused approximately 121,759 lines of imported violative medical products. However, FDA has focused examinations on COVID-19 relief supplies to ensure compliant products are expedited while maintaining our commitment to refusing imported medical products that are unsafe, misbranded, unapproved, counterfeit, or may cause serious illness or injury to the public. In fact, our import investigators have evaluated donations of shipments destined for the Federal Emergency Management Agency (FEMA) and met the first vaccines (Pfizer Belgium) on their arrival into the United States in December 2020 to ensure proper transport, storage, and reconciliation of products, and also assisted with expediting the importation of other compliant vaccine-related shipments as well as other COVID-19 necessities.
Despite generally pausing domestic and foreign surveillance inspections in March 2020 to safeguard the health and well-being of our staff, as well as employees at facilities we inspect, our investigators continued to conduct mission critical inspections both domestically and abroad to ensure FDA-regulated industries were meeting applicable FDA requirements. In July 2020, FDA resumed prioritized domestic inspections. To arm our investigators with the most reliable and accurate information, FDA developed a rating system to assist in determining when and where it was safest to conduct prioritized domestic inspections until we resumed standard inspectional operations for domestic surveillance inspections in July 2021.
On May 5, 2021, FDA issued a report titled, “Resiliency Roadmap for FDA Inspectional Oversight,”3 outlining the Agency’s inspectional activities during the COVID-19 pandemic and its detailed plan to move toward a more consistent state of operations, including FDA’s priorities related to this work going forward. The report was updated on November 22, 2021.4
The report described our oversight work during the pandemic and outlined the inspectional activities that the Agency had postponed due to travel restrictions or inability to ensure the safety of our workforce or the workforces within the industries the Agency regulates. The report also outlined the number of mission-critical inspections FDA completed during that time, such as inspections of facilities for which there was a drug shortage, inspections needed for the approval of novel drugs or drugs related to the potential treatment of COVID-19, support of pre-market and pre-license applications, and response to foodborne disease outbreaks or other food safety risks such as food contaminated with pathogens.
Additionally, the Resiliency Roadmap outlines FDA’s continued, successful use of alternative tools and approaches where inspections are not feasible, including remote assessments (e.g. requests to regulated establishments to remotely view records) and remote interactive evaluations that include remote livestreaming video of operations, teleconferences, or screen sharing, and leveraging information from trusted regulatory partners. For example, FDA made over 1,300 requests to human and animal drug and biological product manufacturers to remotely view records, to support on-time regulatory decision actions. Our review of records requested under section 704(a)(4) of the Federal Food, Drug, and Cosmetic Act supported more than 300 approval recommendations for new or abbreviated drug applications, as well as support for authorization decisions for EUA requests, potentially allowing new products to come to market and provide access to lower cost generic drugs to patients more quickly than may have otherwise been possible.
Notably, FDA’s bioresearch monitoring program staff have conducted more than 130 remote assessments that were directly used in application decisions.5 The new tool was incentivized for and supported by industry and continues to provide the Agency with valuable information to assist with risk-based targeting for inspections. FDA recognizes that remote approaches do not replace physical on-site inspections, and that there are situations where only an on-site inspection is appropriate, based on risk and history of compliance with FDA regulations.
The Resiliency Roadmap further outlined the ongoing steps the Agency is taking to resume standard operational levels of inspection activities, including how it intends to prioritize domestic and foreign inspections that could not be performed during the pandemic. On July 1, 2021, FDA transitioned to standard operations for domestic surveillance inspections and other prioritized operational work through the end of September 2021. As noted in the updated November 2021 report, FDA has exceeded the goals for completing domestic surveillance inspections that were detailed in the May Resiliency Roadmap. We also exceeded our performance goal related to following up on previous inspections classified as official action indicated (OAI). Additionally, of the more than 13,500 applications for medical product approval or authorization received between March 2020 and September 2021, only 68 applications had been delayed due to the inability to conduct inspections — the vast majority of these applications are not deemed mission-critical. From the time FDA first issued the Resiliency Roadmap in May to September 2021, the FDA has been able to make decisions on nearly half of the 68 applications.
When planning surveillance inspections, the Agency is prioritizing higher-risk establishments. For example, a sterile manufacturing site that has not been previously inspected and is making narrow therapeutic index drugs would likely be deemed a higher risk than a site that had a well know inspectional and compliance history that is making over-the-counter solid oral dosage form drugs. This means that postponed inspections will be prioritized based on risk and conducted over a longer period of time, ultimately increasing the amount of time between inspections of certain lower-risk facilities in order to focus on products that present the greatest risk to public health.
The Agency launched a multi-year modernization effort in July 2021 to further transform our data enterprise platforms and cross-program interoperability infrastructure to better support innovation related to its regulatory oversight role. This includes adopting technology to support regulatory assessments to improve our remote receipt, review, and analysis of industry data and records, and improve remote interactions with industry entities to be easier, more efficient, more consistent, and more secure. This modernization effort includes a review of inspectional approaches using next-generation assessment technologies and improvements. FDA established an Agency-wide Inspectional Affairs Council (FIAC) that provides coordination of inspection approaches and assessment processes. The Agency intends to share more information on these efforts as this work progresses. FDA will continue to leverage and maximize every available tool and resource to meet its regulatory oversight responsibilities, while achieving optimal public health outcomes.
Compliance and Enforcement
FDA exercises its regulatory authority by, among other things, issuing warning letters and by pursuing civil and criminal enforcement actions against firms and individuals who do not comply with regulatory requirements, including those distributing unapproved products with false or misleading claims that the products prevent, treat, mitigate, diagnose, or cure COVID-19. In March 2020, FDA launched Operation Quack Hack, which leverages the Agency’s expertise and advanced analytics to protect consumers from fraudulent medical products, including unproven cures, illegitimate test kits, and substandard or counterfeit respirators. FDA has sent hundreds of abuse complaints to domain name registrars and internet marketplaces. The Agency also has sent more than 260 warning letters to sellers of unproven products claiming to treat or cure COVID-19. Working with the Department of Justice (DOJ), FDA has sought and obtained preliminary injunctions that require defendants to halt the sale of unproven products claiming to treat or prevent COVID-19, including one product, “Miracle Mineral Solution,” that, when used as directed, is equivalent to industrial bleach. In addition, since the start of the COVID-19 pandemic, FDA has issued 17 warning letters to owners and/or operators of illicit internet pharmacy websites that offer for sale unapproved and misbranded drugs purported to treat COVID-19 to U.S. consumers.
In addition, ORA’s Office of Criminal Investigations (OCI), working with other federal and local law enforcement agencies, has conducted criminal investigations involving unproven COVID-19-related products. In one such example, OCI investigated a physician who attempted to profit from the pandemic by marketing and selling an unproven COVID-19 treatment. The physician marketed and sold treatment kits—which included hydroxychloroquine—as a cure for COVID-19. In July 2021, the physician pleaded guilty to, among other things, trying to smuggle hydroxychloroquine into the United States to sell in his COVID-19 “treatment kits.” In another case, OCI investigated an individual who attempted to import approximately 1,000 unlawful COVID-19 test kits from China, which were intercepted at a FedEx facility in Memphis, Tennessee. As a result of OCI’s investigation, the individual pleaded guilty in October 2021 to a felony smuggling charge. OCI also has conducted criminal investigations to bring to justice those who tamper with COVID-19 vaccines. For example, OCI investigated a hospital pharmacist who tampered with COVID-19 vaccine doses at a Wisconsin hospital where he worked. On two successive nights, the pharmacist purposefully removed a box of COVID-19 vaccine vials from the hospital’s refrigeration unit intending to render the vaccines inert and no longer effective. Before the full extent of his conduct was discovered, 57 people received doses of the vaccine from these vials. In January 2021, the pharmacist pleaded guilty to two counts of attempting to tamper with consumer products with reckless disregard for the risk that another person will be placed in danger of death or bodily injury. He has been sentenced to three years imprisonment, followed by three years of supervised release, and he must pay approximately $83,800 in restitution to the hospital.
In addition, FDA investigators remain on the front lines at ports of entry, quickly examining, reviewing, and sampling import entries, and refusing admission of violative products where appropriate. We protect the supply chain in two equally critical ways: first, we help ensure safe products are coming in; and second, that illegal, dangerous, and fraudulent products do not get into the country. These efforts include partnering with U.S. Customs and Border Protection (CBP) in establishing satellite laboratories at selected International Mail Facilities (IMFs) with scientists using state-of-the-art screening tools to rapidly identify unapproved, counterfeit and illicit products.
In March 2020, OCI, with the help of domestic law enforcement partners and foreign counterparts in the United Kingdom, led the investigation of fraudulent COVID-19 “treatment kits” that were falsely declared as “water treatment.” Import examination of these shipments found misbranded “kits” intended to treat COVID-19. As a result of this investigation, a British national was charged and arrested for shipping mislabeled and unapproved products. In May 2020, FDA worked with CBP to intercept several shipments of counterfeit facemasks, with the result that they were refused and destroyed before entering U.S. commerce.
FDA also has taken steps to address hand sanitizer products that pose safety concerns, such as products that do not meet the required ethanol or isopropanol levels or that contain or may contain toxic ingredients like methanol or 1-propanol. FDA has tested several hundred products using field-based and laboratory-based tools and found more than a hundred violative products. FDA also has taken steps to help ensure that these dangerous or subpotent products do not enter domestic commerce, including coordinating with CBP to identify such products, and we have listed products made by more than 65 manufacturers on import alert. FDA also placed all alcohol-based hand sanitizers from Mexico on a countrywide import alert to help stop products from entering the U.S. that appear to be in violation until the Agency is able to review the products. That action marked the first time the FDA has issued a countrywide import alert for any category of drug product.
Medical Product Supply Chain
FDA monitors and responds to worldwide demand and supply chain disruptions for medical products caused by the COVID-19 pandemic.6 We work closely with manufacturers, within our current resources and authorities, to help ensure they continue to notify the Agency of any permanent discontinuance or interruption of drug (human and animal), biological product, and device manufacturing in a timely manner and, as noted in FDA’s Fiscal Year (FY) 2022 budget, we are working to better position the Agency and our health care system to assure a strong domestic supply chain in future emergencies.7
This is especially important as the COVID-19 pandemic has exposed major vulnerabilities in the supply chain that FDA continues to face as it works to help ensure access to the treatments and devices that patients and healthcare providers need.
In addition to our usual communications with drug manufacturers, we work closely with healthcare and pharmacy systems, hospitals, providers, and others on the frontlines of COVID-19 patient care to identify problems with access to critical care drugs used to treat COVID-19.
FDA understands the significant impact shortages can have on patient care and we are using our authorities to help prevent and alleviate disruptions. When we identify a shortage, we react swiftly to help mitigate the impact to U.S. patients and health care professionals, and quickly share that information with the public. Restoring and increasing the supply of approved drugs has been the agency’s priority. In addition, where necessary, FDA has issued temporary policies during the COVID-19 emergency to respond to reports from hospitals of increased demand and interruptions in supply, some of which have not resulted in a drug shortage but caused concern about continuing access to drugs to support hospitalized patients with COVID-19. We issued temporary policies for outsourcing facilities registered with FDA and pharmacists in state-licensed pharmacies or federal facilities, regarding the compounding of certain drugs used for hospitalized patients with COVID-19. The Agency has published guidances to help applicants and manufacturers provide FDA with timely and informative notifications about changes in the production of certain human drugs, including biological products, and certain animal drugs. We urged the timely submission of these notifications, which may assist in our efforts to prevent or mitigate shortages of such products. In addition, section 503B(a)(2)(A) of the FD&C Act permits outsourcing facilities to use bulk drug substances to compound drug products that appear on the drug shortage list in effect under section 506E of the FD&C Act at the time of compounding, distribution, and dispensing, when all conditions of section 503B are met.
Our experience with COVID-19 demonstrates that a strong domestic supply chain depends on a resilient supply chain for medical devices as well. Indeed, multiple entities – across both the public and private sector – collectively have important roles to play in strengthening the domestic medical device supply chain. FDA can play a critical role in identifying and preventing shortages for devices, because the Agency not only reviews and authorizes these products, but has unique, collaborative relationships that allow direct engagement with device manufacturers, patients, distributors, healthcare organizations, and other stakeholders. Even before the pandemic hit the U.S., there were disruptions in the supply chain due to higher demand for devices in other nations where COVID-19 was already prevalent and shutdowns in locations from which supplies were sourced. As a result, FDA began shortage mitigation activities for medical devices in January 2020 before the PHE was declared in the U.S., and two months before a pandemic was declared worldwide. At that time, the Agency did not have any dedicated funding or explicit authority regarding prevention or mitigation of medical device or animal drug shortages. The Agency lacked dedicated staff necessary to mitigate supply chain disruptions and/or shortages. Nevertheless, the Agency took several actions to rapidly respond to supply chain needs, including reassigning over 130 staff to perform shortages work across CDRH and contacting over 1,000 manufacturing facilities in 12 countries in just a few weeks’ time to get as much information as possible about critical devices. However, because the Agency lacked any explicit shortages authority at this time, only about one-third of facilities that were contacted responded even in part to CDRH requests because response was voluntary. This lack of explicit authority, staff and supply chain information significantly hampered our efforts to mitigate and prevent shortages at the outset of the pandemic.
On March 27, 2020, the CARES Act was signed into law. The CARES Act gave FDA, for the first time, authority related to device shortages (see section 506J of the FD&C Act). The enactment of the CARES Act at the height of the initial pandemic response gave some authority to help prevent or mitigate medical device shortages during the public health emergency. Specifically, section 506J of the FD&C Act requires manufacturers to notify FDA of a permanent discontinuance in or interruptions in the manufacture of certain devices that are likely to lead to a meaningful disruption in supply of that device in the United States during, or in advance of, a public health emergency. Section 506J also requires FDA to maintain a publicly available list of devices the Agency has determined to be in shortage, as well as devices that have been discontinued. The Agency is also directed to, as it deems appropriate, prioritize and expedite inspections and review of premarket submissions to help alleviate the supply chain constraint.
Since receiving this new authority, during the pandemic response, FDA has among other actions:
- issued guidance on submitting notifications to FDA during the COVID-19PHE and updated the guidance to provide better assistance based on stakeholder feedback;
- published a list of device types it has determined to be in shortage at this time, as well as a list of devices for which we have been notified by manufacturers the device has been permanently discontinued;
- created the infrastructure around receiving, processing, and analyzing notifications to determine devices that are in shortage and publicly post a list of the devices that FDA has determined to be in shortage;
- worked with manufacturers to plan for an expedited device premarket submission, which allows us to increase device availability for products that are most needed.
In addition to the implementation of the new device shortages authorities, FDA has conducted horizon scanning to assess demand for devices needed to respond to the pandemic, including PPE, ventilators, diagnostic supplies, infusion pumps, and non-contact infrared thermometers; and established a rapid response team, working with field personnel to address fraudulent imports. The Agency has likewise worked to prevent and mitigate shortages of testing supplies. For example, FDA collaborated with U.S. Cotton, one of the world’s largest manufacturers of cotton swabs, to develop and produce a polyester-based Q-tip-type swab for testing. FDA also collaborated with laboratories and clinical investigators validating potential alternative sources of control materials, transport media, and swabs. As individual developers validated these alternative components, FDA requested their permission to share their findings publicly so that others could benefit, and we posted these alternatives on our website. In this way, FDA has been serving as a clearinghouse for scientific information that the entire community can leverage to mitigate shortages and increase testing capacity. FDA continues to post this information on a rolling basis on an FAQ website so that labs have access to the latest information regarding alternative controls, transport media, extraction, instruments, and swabs.
FDA continues to work to implement and operationalize the new device shortage authority and utilizing one time funding from COVID supplementals, stand up a new state-of-the-art Resilient Supply chain and Shortages Prevention Program (RSCSPP). Medical device shortages not only put patients in harm’s way but also jeopardize our health care workers on the front lines, during PHEs like the COVID-19 pandemic and every day in our health care system. Moreover, device shortages disproportionately affect at-risk populations and exacerbate health disparities. For these reasons, FDA continues to do all it can within its current authorities and resources to mitigate shortages and supply chain interruptions for COVID-19 and within the U.S. health care system generally.
Congress has acknowledged the importance of FDA’s work on shortages in our health care system and we want to continue working with this Committee and others to make sure FDA has the resources and authorities needed to ensure U.S. patients and health care providers have the medical products they need each day. To ensure the U.S. is properly prepared now, and in the future, we must take action to secure our medical device supply chain, including related materials, parts, and components. The FDA recognizes that this will take resources and expanded authority.
Conclusion
FDA continues to advance its mission to protect and promote public health by helping to ensure the safety of human and animal food, and the safety and effectiveness of medical products. We take our public health mandate very seriously and will continue to work each day to help end this pandemic. We continue to communicate with the American public and make regulatory decisions based on data and sound science. I look forward to continuing to work with the Committee on these efforts and thank you again for the opportunity to testify today.
- 1https://www.fda.gov/media/139638/download
- 2https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-frequently-asked-questions
- 3https://www.fda.gov/media/148197/download
- 4https://www.fda.gov/media/154293/download
- 5https://www.fda.gov/regulatory-information/search-fda-guidance-documents/remote-interactive-evaluations-drug-manufacturing-and-bioresearch-monitoring-facilities-during-covid
- 6https://www.whitehouse.gov/wp-content/uploads/2021/06/100-day-supply-chain-review-report.pdf
- 7FDA Fiscal Year 2022 Justification of Estimates for Appropriations Committees (https://www.fda.gov/media/149616/download)