CardioMEMS HF System - P100045/S056
This is a brief overview of information related to FDA's approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED and product labeling for more complete information on this product, its indications for use, and the basis for FDA's approval.
Product Name: CardioMEMS HF System
PMA Applicant: St. Jude Medical (now Abbott)
Address: 387 Technology Circle NW, Suite #500, Atlanta, GA 30313
Approval Date: February 18, 2022
Approval Letter: Approval order
What is it?
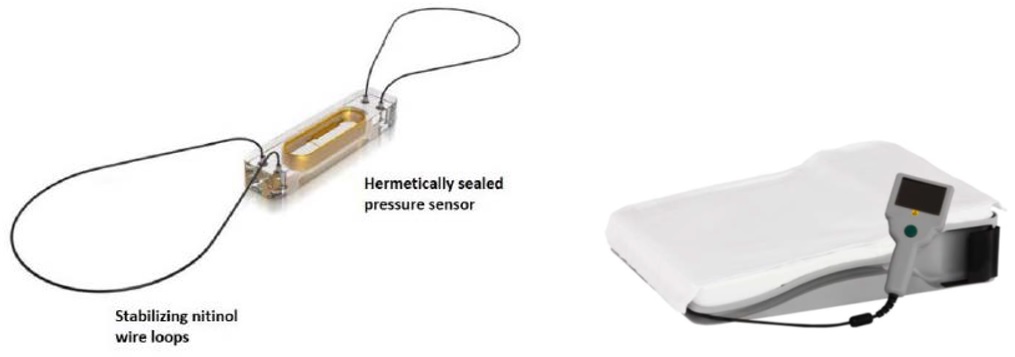
The CardioMEMS HF System wirelessly measures and monitors pulmonary artery (PA) pressure and heart rate for patients with heart failure. The system consists of an implantable pulmonary artery (PA) sensor, delivery system, and patient electronics system.
How does it work?
The CardioMEMS HF implantable sensor is delivered to the pulmonary artery using a long, thin, flexible tube called a transvenous catheter during a minimally invasive heart procedure. The sensor monitors the pressure of blood flowing through the pulmonary artery. The patient uses the Patient Electronics System to take a daily reading of pressure measurements from the sensor. The system then sends the reading information to the health care provider. Based on this data, the health care provider can make any needed adjustments to the treatment plan or medicines the patient receives.
When is it used?
The CardioMEMS HF System is indicated to wirelessly measure and monitor pulmonary artery pressure and heart rate for patients with heart failure.
The system was previously approved for use in patients with more severe heart failure (New York Heart Association class III) who had been hospitalized for heart failure in the last year.
This new approval expands the indications for use to include people with less severe (New York Heart Association class II) heart failure and for those who have increased levels of a substance made by the heart (natriuretic peptides) that can indicate their heart failure is getting worse.
What will it accomplish?
Health care providers can use information from the CardioMEMS HF system to make treatment decisions and manage a patient's heart failure, possibly helping these patients get care sooner and avoid heart failure-related hospitalizations. In a clinical study that included 1,000 people with the device implanted, patients had fewer heart failure-related hospitalizations when doctors were able to monitor their patients' pulmonary artery pressure data.
When should it not be used?
The CardioMEMS HF System should not be used in people who are unable to take blood-thinning (anticoagulant) or anti-clotting (antiplatelet) medications for one month after receiving the implant.
Additional information (including warnings, precautions, and adverse events)
- Summary of Safety and Effectiveness Data (SSED)
- Labeling (Patient System Guide)
- Labeling (User's Manual)
- PMA database entry