Metal-on-Metal Hip Implant Systems
Metal-on-Metal (MoM) hip implants consist of a ball, stem and shell, all made of metal materials. MoM hip implants were designed to offer the following benefits:
Less total material being removed from the ball and socket when rubbing against each other when compared to other hip implant systems
- Decreased chance of dislocation when the ball of the thighbone (femur) slips out of its socket in the hip bone (pelvis)
- Decreased chance of device fracture
- There are two types of MoM hip systems:
Total hip replacement implant
- Total resurfacing hip implant
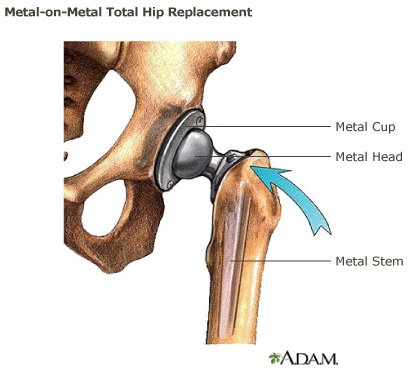
Metal-on-Metal Total Hip Replacement Implant
Metal-on-Metal total hip replacement systems consist of a metal ball (femoral head), a metal femoral stem in the thighbone, and a metal cup in the hip bone (acetabular component). The ball attaches to the taper of the stem.
On February 18, 2016, the FDA issued a final order requiring manufacturers to submit a premarket approval (PMA) application for two types of metal-on-metal MoM total hip replacement devices: the hip joint metal/metal semi-constrained with a cemented acetabular component and the hip joint metal/metal semi-constrained with an uncemented acetabular component.
As summarized in these final orders and given the known risks, the FDA believed there was insufficient evidence to conclude that general controls in combination with special controls would provide reasonable assurance of the safety and effectiveness of these devices. The FDA determined that these devices should remain Class III (higher risk) devices and PMA applications were to be filed with the Agency by May 18, 2016, if a manufacturer wanted to continue marketing their metal-on-metal total hip replacement devices or market new metal-on-metal total hip replacement devices.
To date, there are no approved PMA applications for metal-on-metal total hip replacements, and these devices are not currently legally marketed. However, some patients who had hip replacement prior to May 18, 2016 may have received a metal-on metal hip. Information for patients and health care providers about metal-on-metal hip implants is provided in this websection.
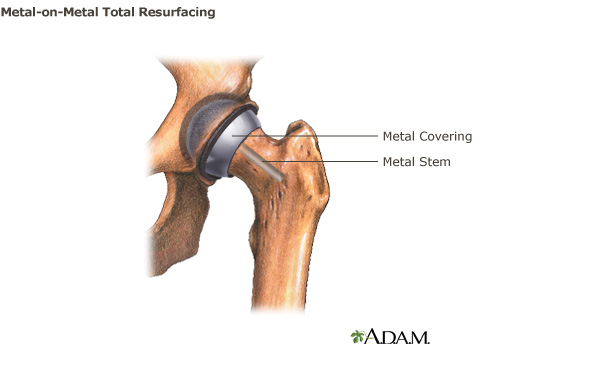
Metal-on-Metal Total Resurfacing Hip Implant Systems
Metal-on-Metal total hip resurfacing systems consist of a trimmed femoral head capped with a metal covering. Any damaged bone and cartilage within the socket are removed and replaced with a metal acetabular component.
To date, there are two FDA-approved metal-on-metal total hip resurfacing systems through the premarket approval (PMA) program. In each approved premarket application, the manufacturer demonstrated the safety and effectiveness of their metal-on-metal total hip resurfacing system through non-clinical and clinical performance data. Links to the Summary of Safety and Effectiveness Data for both of the approved pre-market approvals are available below:
- Corin USA Limited Cormet Hip Resurfacing System
- Smith & Nephew, Inc. Birmingham Hip Resurfacing (BHR) System