Online 506J Notification Submission Methods: Frequently Asked Questions
This page responds to common questions on online 506J notification submission methods, including the webform and spreadsheet template, under section 506J of the Federal Food, Drug, and Cosmetic (FD&C) Act.
On this page:
Webform FAQs
Q. How do I submit notifications using the webform? Where can I find help if I have trouble using the webform?
A. Please see the How to Use the FDA 506J Notification Webform for more information on submitting information through the webform, troubleshooting, and answers to frequently asked questions on the webform.
Q. Is the information I submit using the webform secure?
A. The FDA 506J Notification webform complies with Hypertext Transfer Protocol Secure (HTTPS) messaging standards and uses digital certificates for secure communication.
Q: What is the purpose of the "Voluntarily Indicate No Current Interruption or Permanent Discontinuance" option on the online webform?
A: If a manufacturer is currently not experiencing an interruption or permanent discontinuance in manufacturing, they may voluntarily indicate this to the FDA selecting this option and filling out the form.
Q. Are there ways other than the webform to submit a 506J notification?
A. Manufacturers may submit using the webform. However, there are alternate methods to submit a 506J notification. For example, if you do not utilize the webform, manufacturers may email their information to CDRHManufacturerShortage@fda.hhs.gov and begin the email subject line with the word "Notification." For more information, see Supply and Shortages of Medical Devices: Frequently Asked Questions.
Q. I'm having trouble loading the form in my web browser. Are there certain web browsers that work better than others?
A. Please use Chrome, Microsoft Edge, or Firefox to fill out the webform.
Q. Can I see what information I have submitted previously?
A. No, once the webform is submitted, the information is sent to the FDA and is not saved within the webform. If you have a question about the information you submitted, please email CDRHManufacturerShortage@fda.hhs.gov and include "Question" in the subject line of the email.
Q. Can I use multiple methods to submit information using the webform?
A. Yes, you can submit notifications through any or all of the three methods in the webform (for example, webform, spreadsheet, voluntary notification). Once you've completed and submitted notification using one method, you will be redirected to the webform home page, where you can select a different method.
Q. How do I know if the FDA received my webform submission?
A. Once you have submitted a 506J notification through the webform, a confirmation email will be sent to the identified contact's email address. To inform possible mitigation efforts, the FDA may follow up with manufacturers or conduct targeted outreach where an interruption is cross-cutting or may have the potential to impact users. However, you will not routinely hear from the FDA once the FDA has analyzed the information.
The FDA will publicly communicate updates to the device shortage list and device discontinuance list. Any information that is trade secret or confidential information will be treated as such, consistent with section 552(b)(4) of title 5, United States Code, section 1905 of title 18, United States Code, section 520(c) of 21 USC 360J(c), and other applicable laws.
Q. What if I want to provide the FDA with additional information than is required or requested? How can I submit that information?
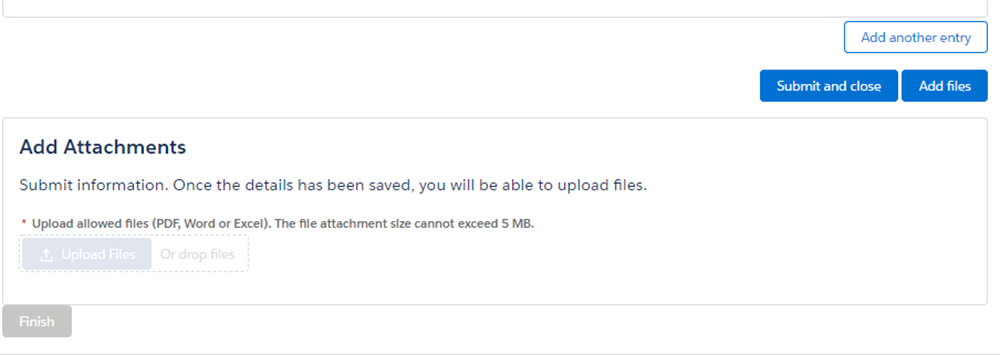
A. When using the webform, you may attach additional documents if there is additional information you wish to provide to the FDA. Select "Add files" at the bottom of the page and the option to add attachments will become available.
Q. How do I submit a 506J notification for a product code that is not available on the webform?
A. If your product code is not available, please contact the FDA for further instruction at CDRHManufacturerShortage@fda.hhs.gov and include "Product Code" in the subject line. Include your name, organization, and contact information and an FDA team member will contact you with instructions on how to submit your notification.
Q. How do I submit a 506J notification for an FEI that is not available on the webform?
A. If you are having trouble locating your FEI (FDA Establishment Identifier), see How to Determine FEI, product code, and submission number for information on how to locate your firm's establishment information. If you are still unable to locate your establishment's FEI number in the webform, please email CDRHManufacturerShortage@fda.hhs.gov and include "Question" in the subject line.
Q. There are three different notification options on the webform. How do I know if I have a "small" or "large" amount of FEI-product codes to enter?
A. Manufacturers may submit 506J notifications using the method that is most convenient for you.
Generally, for a manufacturer having between 5 and 10 FEI-product code combinations, submitting 506J notifications directly through the webform may be easiest.
For a manufacturer having over 10 FEI-product code combinations, the spreadsheet template may be a more convenient method to submit a 506J notification. You can then submit it to the FDA in your initial notification, as well as use it to collect any revised information for update notifications.
Note: The webform is updated weekly to include new information from Registration & Listing, but this update does not affect the format of the spreadsheet.
Q. I ran out of character space in a box. How can I add more context to my answer?
A. If you have exceeded the character limit in a text box field, an additional file can be attached to your submission that contains any additional information. Please find the option to attach a file at the bottom of the form.
Q. What if the options for the reason for interruption don't fully explain my situation?
A. You have the option to select several reasons for the discontinuance or interruption as well as an option of "Other." An open text field is below this question where you can explain the reason for the discontinuance or interruption if you feel the options don't explain your situation. Additionally, there is an option to attach a file at the end of your submission.
Q. Is there a way of notifying the FDA that I do not currently have an interruption or permanent discontinuance?
A. Yes. If you are not currently experiencing an interruption or discontinuance in manufacturing, you may voluntarily notify the FDA using the "Voluntarily Indicate No Current Interruption or Permanent Discontinuance" option on the webform.
Q. Where can I find more information about 506J notifications?
A. See Medical Device Supply Chain and Shortages for more information about 506J notifications. For answers to frequently asked questions about the device shortage list, who to contact, or 506J Notifications in general, please see Supply and Shortages of Medical Devices: Frequently Asked Questions.
Spreadsheet Template FAQs
Q. How do I submit large numbers of notifications using the spreadsheet? Where can I find help if I have trouble entering information in the spreadsheet?
A. Please see the How to Use the 506J Notification Spreadsheet Template for more information on submitting information through the webform, troubleshooting, and answers to frequently asked questions on the webform.
Q. Can I submit a spreadsheet using the webform that does not use the FDA spreadsheet template?
A. No. Spreadsheet files that are not formatted in the FDA spreadsheet template will not be able to be processed correctly when submitting online using the webform.
However, you may email a spreadsheet to CDRHManufacturerShortage@fda.hhs.gov and begin the email subject line with the word "Notification."
If you are experiencing problems with the FDA template spreadsheet, email CDRHManufacturerShortage@fda.hhs.gov and include "Question" in the subject line.