SonarMed Inc. Recalls Airway Acoustic Sensors Due to a Restricted Inner Diameter of Airway Causing Suction Catheter Passage Difficulty
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Recalled Product
- Product Names: SonarMed Airway acoustic sensors
- Product Codes: OQU
- Model Numbers: AW-S025, AW-S030, AW-S035
- Distribution Dates: October 12, 2022 to August 11, 2023
- Devices Recalled in the U.S.: 1,800
- Date Initiated by Firm: March 21, 2024
Device Use
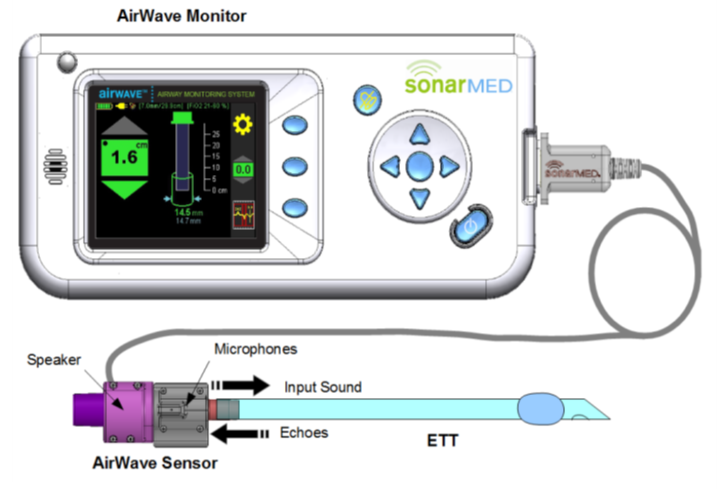
The SonarMed airway monitoring system (AMS) is comprised of a SonarMed monitor (monitor) that is used in conjunction with a single-patient use SonarMed Sensor (Sensor), connected via an external cable connection, and software that operates the Monitor and Sensor.
When in use, the SonarMed AirWave airway sensor is placed in-line between the ventilator circuit and the proximal end of the endotracheal tube (ETT) of a patient who is connected to a ventilator. The Sensor replaces the standard 15-mm connector (also known as the “hub”) that comes with the ETT and utilizes acoustic reflection technology to provide real-time information to clinicians. This includes the baseline location of the ETT tip, estimation of passageway size around the tip, detection of ETT movement, and identification of occlusions or obstructions.
The SonarMed airway monitoring system is intended for use by trained medical professionals to support the management of artificial airways in hospital settings such as intensive care units, operating rooms, emergency departments, and during intrahospital transport. It is meant to complement standard clinical practices and is not intended as a standalone diagnostic tool. The system is suitable for use across a wide range of patients, including neonates, infants, children, adolescents, and adults, covering a size range from 2.5mm to 9.0mm for endotracheal tubes.
Reason for Recall
SonarMed Inc. is recalling SonarMed Airway acoustic sensors due to a restricted inner diameter of SonarMed Airway resulting in difficulty passing a suction catheter through the sensor (2.5mm, 3.0mm, and 3.5mm).
Use of the affected SonarMed Sensors may cause serious adverse health consequences, including delays in treatment, low oxygen levels (hypoxia), air leaking into the chest cavity (pneumothorax), not enough air reaching the lungs (hypoventilation), harm to the tissues, slow heart rate (bradycardia), or even breathing problems leading to respiratory failure.
There have been 1 reported injury and no reports of death.
Who May be Affected
- Health care providers who use the SonarMed airway monitoring system to support the management of artificial airways.
- People who receive care with the SonarMed airway monitoring system is intended for use by trained medical professionals to support the management of artificial airways.
What to Do
On March 25, 2024, Medtronic, on behalf of SonarMed Inc. sent all affected customers an Urgent Medical Device Recall notice.
The letter requested customers to:
- Discontinue use of affected sensors and quarantine all SonarMed Airway System products.
- Return all quarantined SonarMed airway sensors to Medtronic.
- Please contact rs.covidienfeedbackcustomerservice@medtronic.com for the Return Goods Authorization (RGA). Credit for the returned product will be issued based on the RGA number.
- Complete and return the included Customer Confirmation Form to rs.gmbmitgfca@medtronic.com.
- Share this information anywhere the SonarMed Airway sensors may have been transferred or distributed.
Contact Information
Customers in the U.S. with questions about this recall should contact Medtronic Representative or Customer Service at 800-962-9888, Option 2.
Additional Resources:
- Class 1 Device Recall SonarMed AirWave (fda.gov)
- Class 1 Device Recall SonarMed AirWave (fda.gov)
- Class 1 Device Recall SonarMed AirWave (fda.gov)
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.