Insulet Corporation Recalls Omnipod 5 Android App due to a Software Error
Please be aware, this recall is a correction, not a product removal.
The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
Recalled Product
- Product Names: Omnipod 5 App (on compatible Android smartphones)
- Product Codes: QFG
- Software Version Number: 1.1 - 1.2.3
- Distribution Dates: April 20, 2023 to December 20, 2023 (For software versions 1.1-1.2.3)
- Devices Recalled in the U.S.: 28,919
- Date Initiated by Firm: November 7, 2023
Device Use
The Omnipod 5 App, available for compatible Android smartphones, is part of the Omnipod 5 System which is an automated insulin delivery system designed to administer insulin to people 2 years of age or older with type 1 diabetes. The Omnipod 5 System includes the Omnipod 5 ACE Pump, the Omnipod 5 App, and a compatible integrated continuous glucose monitor (the Dexcom G6). The Omnipod 5 App uses "SmartAdjust" technology to help adjust insulin delivery based on real-time and future blood sugar levels. The SmartBolus Calculator computes recommended bolus doses. It considers user-inputted carbohydrates, recent sensor glucose (or fingerstick reading), glucose change rate (if available), insulin on board (IOB), and customizable correction factor, insulin-to-carbohydrate ratio, and target glucose value.
The recalled Omnipod 5 App is an Android-based software that is provided either on a locked-down Controller or is available to download through Google Play on compatible Android smartphones. This app controls the Omnipod 5 Automated Insulin Delivery System. It is used to activate/deactivate Pods, display alerts/alarms, and send insulin delivery commands for execution to the Pod.
Reason for Recall
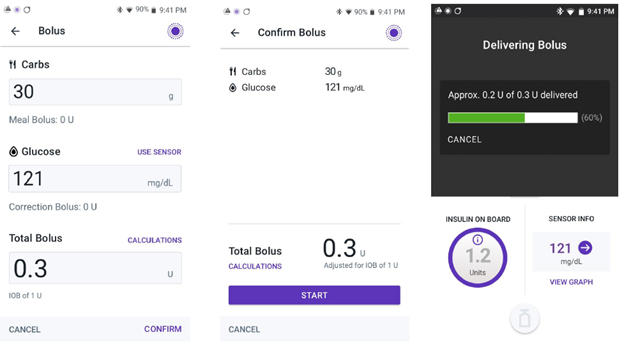
Insulet Corporation is recalling the Omnipod 5 App for compatible Android smartphones due to a software error that occurs when the user enters a bolus amount less than 1 unit without putting a leading zero before the decimal point. Due to this error, when entering a bolus dose of insulin less than 1 unit, the SmartBolus calculator does not register the decimal point if it is the first character entered.
For example, if a user enters ".2" intending to request a 0.2 units insulin bolus, the app would not register the decimal point and would display 2 units as the requested insulin bolus instead. Or if a user enters ".20” units, the app could display 20 units as the requested insulin bolus instead. This failure to recognize the decimal point when entered without a leading zero may lead to the app giving too much insulin, anywhere from 10 times to up to 100 times the intended amount, if the user does not recognize the error on the calculator screen or the confirmation screen before confirming the dose.
On November 30, 2023 Insulet Corporation sent all affected customers and health care providers an URGENT Medical Device Correction notice. The letter requested customers to follow instructions when changing a suggested bolus and always visually confirm the bolus amount is critical as the system will always deliver this amount.
The firm released a software update v1.2.4 on December 7, 2023 to address and correct the issue. Users should have received a message that an update to the Omnipod 5 App was required and that following December 20, 2023, the application would not be available until they performed the update.
The use of affected product without the software update may cause serious adverse health consequences, including severe hypoglycemia and death.
There have been 2 reported injuries. There have been no reports of death.
Who May be Affected
- People with diabetes that use the Omnipod 5 App, downloaded to their personal Android smartphones from the Google Play store, for the delivery of insulin.
- In the US, this recall does not affect the Omnipod 5 App running on the Insulet-provided Personal Diabetes Manager (PDM) controller.
What to Do
On December 7, 2023, Insulet Corporation sent all affected customers a Medical Device Correction notice.
This letter requested customers to:
- Update the Omnipod 5 Android App to v1.2.4
- Confirm that the displayed insulin bolus amount matches the desired amount when using the Omnipod 5 Android App
Contact Information
Customers in the U.S. with questions about this recall should contact Insulet Customer Care at 1-800-591-3455.
Additional Resources:
How do I report a problem?
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program using an online form, regular mail, or FAX.