Send and Track Medical Device Premarket Submissions Online: CDRH Portal
Update: September 30, 2025
As of October 1, 2025 all De Novo submissions, unless exempted, must be submitted as electronic submissions using eSTAR, as noted in the final guidance: Electronic Submission Template for Medical Device De Novo Requests.
The CDRH Portal updates for PMAs are a step forward in meeting the Medical Device User Fee Amendments 2022 (MDUFA V) commitments of using technology to enhance efficiency and transparency in reviewing industry submissions.
All CDRH-led premarket submission types may be uploaded to the CDRH Portal at any stage of the review process. Official correspondents do not need to send a physical cover letter to the FDA after uploading an electronic submission to the CDRH Portal. Please note that the CDRH Portal cannot receive submission files larger than 4GB or PDF files with attachments larger than 1GB.
On this page:
- Overview
- CDRH Portal: Send Medical Device Premarket Submissions and Small Business Requests Online
- Progress Tracker for Electronic Submissions
- Technical Limitations
- For More Information
Overview
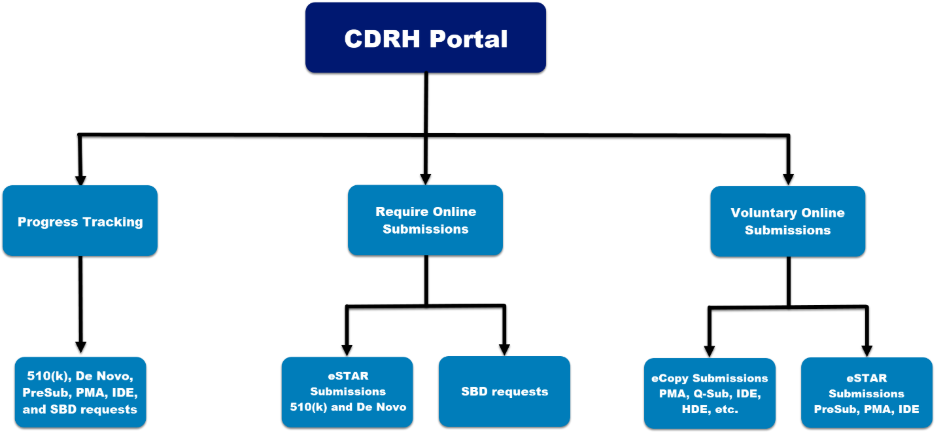
The Customer Collaboration Portal ("CDRH Portal") provides progress tracking and online submission capabilities for CDRH premarket submissions.
The FDA created and improved the CDRH Portal to meet commitments for Medical Device User Fee Amendments (MDUFA) IV and V.
CDRH Portal: Send Medical Device Premarket Submissions and Small Business Requests Online
Anyone can register for an account in the CDRH Portal and send premarket submissions and Small Business Requests online.
Important: CDRH uses Okta for identity verification and single sign-on purposes when users register for a CDRH Portal account. Account registration and password reset requests will come from an email ending in @okta.com.
As of October 1, 2025, all De Novo submissions, unless exempted*, must be submitted as electronic submissions using eSTAR.
As of November 1, 2024, all Small Business Requests must be submitted using the CDRH portal.
As of October 1, 2023, all 510(k) submissions, unless exempted**, must be submitted as electronic submissions using eSTAR.
Submissions and requests received before 4 PM ET on a business day (Monday through Friday, excluding U.S. Federal holidays) will be processed the same day. If received after 4PM ET, the submission will be processed the next business day.
*As noted in the final guidance, Electronic Submission Template for Medical Device De Novo Requests, all De Novo Requests including originals and any supplements and amendments are required to be submitted as electronic submissions, unless exempted in Section VI.A Waivers and Exemptions from Electronic Submission Requirements of the guidance. The electronic submission template, eSTAR, is the only currently available electronic submission template to facilitate the preparation of De Novo electronic submissions.
**As noted in the final guidance, Electronic Submission Template for Medical Device 510(k) Submissions: Guidance for Industry and FDA Staff, all 510(k)s, including Traditional, Special, Abbreviated, and any supplements and amendments are required to be submitted as electronic submissions, unless exempted in Section VI.A Waivers and Exemptions from Electronic Submission Requirements of the guidance. The electronic submission template, eSTAR, is the only currently available electronic submission template to facilitate the preparation of 510(k) electronic submissions.
NOTE: Only CDRH submissions may be sent through the CDRH Portal. For biologic products or devices used in blood establishments, please submit to the Center for Biologics, Evaluation, and Research (CBER).
Progress Tracker for Electronic Submissions
The online progress tracker has a dashboard that displays near real-time submission status. The FDA secures the information about each submission's progress to ensure only the official correspondent or designated delegates* for that submission can view the status.
When you send a CDRH Pre-Submission, 510(k) submission (traditional, special, and abbreviated 510(k)s), De Novo classification request, Premarket Approval (PMA) application, PMA Panel-Track supplement, Investigational Device Exemptions (IDE), or Small Business Request for review, your official correspondent or designated delegates can monitor the FDA’s progress online in a simple, concise format.
The official correspondent for an eSTAR submission is the person identified in the:
- “Contact” section, or as the
- “Primary Correspondent/ Consultant,” if applicable.
The official correspondent for an eCopy submission is the person identified in:
- Section C of CDRH Premarket Review Submission Cover Sheet (Form FDA 3514) or in
- Section B (if Section C is blank on the Cover Sheet form), or
- in the Cover letter, if the eCopy submission does not include a Cover Sheet form.
The official correspondent for a Small Business Request is the person identified in:
- Section I, Box 4 (or Box 8, where applicable) of Form FDA 3602N
* Note: At this time, only the official correspondent can view the status of their Small Business Request. Assignment to a designated delegate will be available in a future update.
What to expect:
- If this is your official correspondent’s first time tracking a submission online, the FDA automatically emails a link to create a login password soon after the FDA receives the submission.
- While the CDRH Portal features online progress tracking for Pre-Submissions, 510(k) submissions, De Novo classification requests, PMA applications, PMA Panel-Track supplements, IDEs, and Small Business Requests, the FDA also formally notifies you of your submission's status by emailing your official correspondent with official actions and requests.
Technical Limitations
The CDRH Portal cannot receive individual files that are:
- larger than 4GB, or
- PDFs with an attachment larger than 1GB.
To submit electronic files that exceed the technical limitations, you can mail the electronic version of your submission to the CDRH Document Control Center (DCC) at this address:
U.S. Food and Drug Administration
Center for Devices and Radiological Health
Document Control Center (DCC) – WO66-G609
10903 New Hampshire Avenue
Silver Spring, MD 20993-0002
For more information on mailing submissions, please read eCopy Medical Device Submissions.
For more information:
- eCopy Program for Medical Device Submissions
- Electronic Submissions
- eSTAR Program
- How to Study and Market Your Device
- Premarket Approval
- Small Business Determination (SBD) Program
Questions?
If you have questions about:
- CDRH Portal, check the Portal Help articles inside the CDRH Portal, or email ccp@fda.hhs.gov
- eCopy, email CDRH-eCopyinfo@fda.hhs.gov
- eSTAR, email eSubPilot@fda.hhs.gov
- Device regulation in general, email DICE@fda.hhs.gov
- Specific premarket submissions, email OPEQSubmissionSupport@fda.hhs.gov
- General Small Business Determination Program information, email DICE@fda.hhs.gov