Human Factors Considerations
Human Factors Engineering
Understanding how people interact with technology and studying how user interface design affects the interactions people have with technology is the focus of human factors engineering (HFE) and usability engineering (UE).
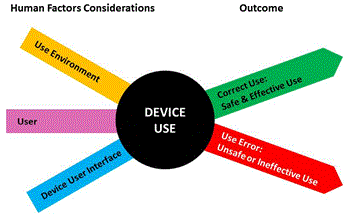
HFE/UE considerations in the development of medical devices involve the three major components of the device-user system: (1) device users, (2) device use environments and (3) device user interfaces. The interactions among the three components and the possible results are depicted graphically in Figure 1.
Figure 1: Interactions among HFE/UE considerations result in either safe and effective use or unsafe or ineffective use.
Device Users
The intended users of a medical device should be able to use the device without making use errors that could compromise medical care or patient or user safety.
Depending on the specific device and its application, device users might be limited to professional caregivers, such as physicians, nurses, nurse practitioners, physical and occupational therapists, social workers, and home care aides. Other user populations could include medical technologists, radiology technologists, or laboratory professionals. Device user populations might also include the professionals who install and set up the devices and those who clean, maintain, repair, or reprocess them. The users of some devices might instead be non-professionals, including patients who operate devices on themselves to provide self-care and family members or friends who serve as lay caregivers to people receiving care in the home, including parents who use devices on their children or supervise their children’s use of devices.
The ability of a user to operate a medical device depends on his or her personal characteristics, including:
- Physical size, strength, and stamina,
- Physical dexterity, flexibility, and coordination,
- Sensory abilities (i.e., vision, hearing, tactile sensitivity),
- Cognitive abilities, including memory,
- Medical condition for which the device is being used,
- Comorbidities (i.e., multiple conditions or diseases),
- Literacy and language skills,
- General health status,
- Mental and emotional state,
- Level of education and health literacy relative to the medical condition involved,
- General knowledge of similar types of devices,
- Knowledge of and experience with the particular device,
- Ability to learn and adapt to a new device, and
- Willingness and motivation to learn to use a new device.
Device Use Environment
The environments in which medical devices are used might include a variety of conditions that could define what is a good user interface design. Medical devices might be used in clinical environments or non-clinical environments, community settings or moving vehicles. Examples of environmental use conditions include the following:
- The lighting level might be low or high, making it hard to see device displays or controls.
- The noise level might be high, making it hard to hear device operation feedback or audible alerts and alarms or to distinguish one alarm from another.
- The room could contain multiple models of the same device, component or accessory, making it difficult to identify and select the correct one.
- The room might be full of equipment or clutter or busy with other people and activities, making it difficult for people to maneuver in the space and providing distractions that could confuse or overwhelm the device user.
- The device might be used in a moving vehicle, subjecting the device and the user to jostling and vibration that could make it difficult for the user to read a display or perform fine motor movements.
Device User Interface
A device user interface includes all points of interaction between the user and the device, including all elements of the device with which the user interacts. A device user interface might be used while user setups the device (e.g., unpacking, set up, calibration), uses the device, or performs maintenance on the device (e.g., cleaning, replacing a battery, repairing parts). It includes:
- The size and shape of the device (particularly a concern for hand-held and wearable devices),
- Elements that provide information to the user, such as indicator lights, displays, auditory and visual alarms,
- Graphic user interfaces of device software systems,
- The logic of overall user-system interaction, including how, when, and in what form information (i.e., feedback) is provided to the user,
- Components that the operator connects, positions, configures or manipulates,
- Hardware components the user handles to control device operation such as switches, buttons, and knobs,
- Components or accessories that are applied or connected to the patient, and
- Packaging and labeling, including operating instructions, training materials, and other materials.