WARNING LETTER
Eonsmoke, LLC MARCS-CMS 592097 —
- Delivery Method:
- VIA UPS and Electronic Mail
- Product:
- Tobacco
- Recipient:
-
Recipient NameKelly L. Zeller
-
Recipient TitleGeneral Manager
- Eonsmoke, LLC
1500 Main Avenue, Suite 2
Clifton, NJ 07011
United States
- Issuing Office:
- Center for Tobacco Products
10903 New Hampshire Avenue
Silver Spring, MD 20993
United States
Kellyeonsmoke@gmail.com
WARNING LETTER
Dear Ms. Zeller:
The Center for Tobacco Products of the U.S. Food and Drug Administration (FDA) has reviewed your submissions to the FDA, our inspection records, the verified Twitter account of Scott Disick (https://twitter.com/scottdisick) and the verified Instagram account of Mia Khalifa (https://www.instagram.com/miakhalifa), which contain labeling and/or advertising for several e-liquid products posted on behalf of Eonsmoke, LLC, as well as the website https://www.eonsmoke.com and the Twitter account of Eonsmoke, LLC, https://twitter.com/eonsmoke. FDA has determined that Eonsmoke, LLC, advertises, imports, offers for sale, and/or distributes electronic nicotine delivery system (ENDS) products and ENDS components and parts, including e-liquids, for commercial distribution in the United States.
Under section 201(rr) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. § 321(rr)), as amended by the Family Smoking Prevention and Tobacco Control Act, these products are tobacco products because they are made or derived from tobacco and intended for human consumption or are any component or part of a tobacco product. Certain tobacco products, including ENDS products and ENDS components and parts, including e-liquids, are subject to FDA jurisdiction under section 901(b) of the FD&C Act (21 U.S.C. § 387a(b)).
New Tobacco Products Without Required Marketing Authorization Are Adulterated and Misbranded
FDA has determined that you import, sell, and/or distribute to customers in the United States the following ENDS products and ENDS components and parts, including e-liquids, without a marketing authorization order:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Salt Nicotine E-Liquid 30mg |
|
|
|
|
|
Nicotine E-Liquid 30mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Salt Nicotine E-Liquid 30mg |
|
|
Salt Nicotine E-Liquid 60mg |
|
|
|
|
|
|
|
|
|
|
The FD&C Act requires premarket review for any “new tobacco product,” which means any tobacco product that was not commercially marketed in the United States as of February 15, 2007, or any modification of a tobacco product where the modified product was commercially marketed in the United States after February 15, 2007 (section 910(a) of the FD&C Act; 21 U.S.C. § 387j(a)). Generally, a marketing authorization order under section 910(c)(1)(A)(i) of the FD&C Act (21 U.S.C. § 387j(c)(1)(A)(i)) is required for a new tobacco product unless (1) FDA issues an order finding the product substantially equivalent to a predicate tobacco product (section 910(a)(2)(A) of the FD&C Act) or (2) FDA issues an order finding the product to be exempt from the requirements of substantial equivalence and you make the required submission under section 905(j)(1)(A)(ii) of the FD&C Act (21 U.S.C. § 387e(j)(1)(A)(ii)). All deemed products that meet the definition of a “new tobacco product,” including ENDS products and ENDS components and parts, such as e-liquids, are subject to the premarket requirements in sections 910 and 905 of the FD&C Act.
FDA has determined that the ENDS products listed in the table above were not commercially marketed in the United States as of February 15, 2007.
These products are required to have premarket review and do not have FDA marketing authorization orders in effect under section 910(c)(1)(A)(i) of the FD&C Act. Therefore, they are adulterated under section 902(6)(A) of the FD&C Act. In addition, they are misbranded under section 903(a)(6) of the FD&C Act because a notice or other information respecting these products was not provided as required by section 905(j) of the FD&C Act (21 U.S.C. § 387e(j)). The introduction into interstate commerce of any tobacco product that is adulterated or misbranded is a prohibited act under section 301(a) of the FD&C Act (21 U.S.C. § 331(a)). Additionally, to the extent that a report was required under section 905(j) of the FD&C Act, the failure to provide such report is a prohibited act under section 301(p) of the FD&C Act (21 U.S.C. § 331(p)).
Modified Risk Tobacco Product Violations
FDA has determined that your ENDS products are adulterated under section 902(8) of the FD&C Act (21 U.S.C. § 387b(8)) because they are modified risk tobacco products sold or distributed without an FDA order in effect that permits such sale or distribution.
Our review of https://www.eonsmoke.com and https://www.faceboook.com/pg/Eonsmoke/about revealed that you sell or distribute ENDS products the label, labeling, or advertising of which represents explicitly and/or implicitly that the products present a lower risk of tobacco-related disease or are less harmful than one or more other commercially marketed tobacco products and/or does not contain or is free of a substance. Specifically, the website https://www.eonsmoke.com includes the claim: “Eonsmoke electronic cigarettes provide you with a premium vaping experience without the thousands of harmful chemicals and additives often found in tobacco cigarettes.” The webpage https://www.faceboook.com/pg/Eonsmoke/about includes the claim: “No tar… No harmful odors that reek.”
A tobacco product is considered a “modified risk tobacco product” under section 911(b)(2)(A)(i) of the FD&C Act (21 U.S.C. § 387k(b)(2)(A)(i)) if its label, labeling, or advertising explicitly or implicitly represents that: (1) the product presents a lower risk of tobacco-related disease or is less harmful than one or more other commercially marketed tobacco products; (2) the product or its smoke contains a reduced level of a substance or presents a reduced exposure to a substance; or (3) the product or its smoke does not contain or is free of a substance. Under section 911(a) of the FD&C Act (21 U.S.C. § 387k(a)), no person may introduce or deliver for introduction into interstate commerce any modified risk tobacco product without an FDA order in effect under section 911(g) of the FD&C Act (21 U.S.C. § 387k(g)). A modified risk tobacco product application under section 911(d) of the FD&C Act (21 U.S.C. § 387k(d)) is required to provide scientific evidence and other information to support issuance of an order under section 911(g) of the FD&C Act (21 U.S.C. § 387k(g)). A product that is in violation of section 911(a) of the FD&C Act (21 U.S.C. § 387k(a)) is adulterated under section 902(8) of the FD&C Act (21 U.S.C. § 387b(8)).
The labeling and/or advertising for your ENDS products, which uses the claims above, represents explicitly and/or implicitly that these products present a lower risk of tobacco-related disease or are less harmful than one or more other commercially marketed tobacco products and /or does not contain or is free of a substance. As such, these products are modified risk tobacco products. Because these products are sold or distributed to customers in the United States without an appropriate FDA order in effect under section 911(g) of the FD&C Act (21 U.S.C. § 387k(g)), these products are adulterated under section 902(8) of the FD&C Act (21 U.S.C. § 387b(8)).
ENDS Products with Advertising that Fails to Include the Required Nicotine Warning Statement are Misbranded
FDA has determined that several ENDS products advertised on behalf of, and by, Eonsmoke, LLC are misbranded under section 903(a)(7)(A) of the FD&C Act (21 U.S.C. § 387c(a)(7)(A)) because the advertising for these ENDS products, in social media posts on your behalf and in social media posts by you, fails to include the required nicotine warning statement.
FDA has also determined that several ENDS products offered for sale or distribution by Eonsmoke, LLC are misbranded under section 903(a)(7)(B) of the FD&C Act (21 U.S.C. § 387c(a)(7)(B) because the advertising for these ENDS products in social media posts on your behalf, in social media posts by you, and on your website fails to include the required nicotine warning statement.
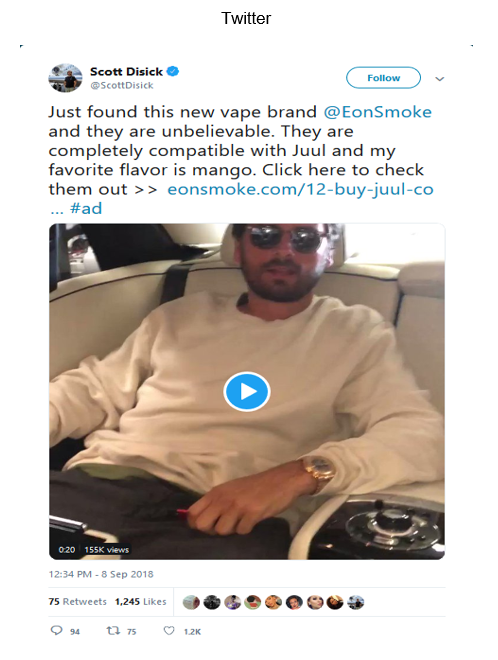
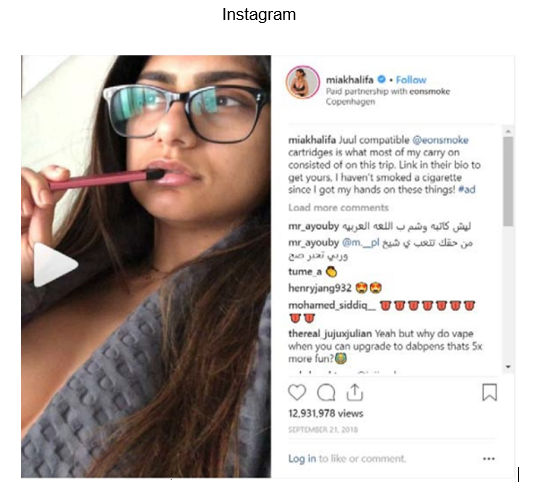
Review of the verified Twitter account of Scott Disick (https://twitter.com/scottdisick) and the verified Instagram account of Mia Khalifa (https://www.instagram.com/miakhalifa) revealed that they contain posts on behalf of Eonsmoke, LLC, dated September 8, 2018 and September 21, 2018, respectively, with advertising for e-liquid products that you manufacture, package, import, distribute, or retail in the United States that does not include the required nicotine warning statement in the manner required by 21 C.F.R. § 1143.3(b), for example: Eonsmoke Pods Mango and Eonsmoke Juul Compatible Pods (see examples below).
Review of the Eonsmoke, LLC Twitter account https://twitter.com/eonsmoke revealed that it contains posts dated August 16, 2018 and August 18, 2018 with advertising for the ENDS products that you manufacture, package, import, distribute, or retail in the United States that does not include the required nicotine warning statement in the manner required by 21 C.F.R. § 1143.3(b), for example: Eonsmoke Salt Nicotine e-liquid products and Eonsmoke Disposable ENDS products.
Review of your website https://www.eonsmoke.com revealed that advertising for ENDS products that you manufacture, package, import, distribute, or retail in the United States does not include the required nicotine warning statement in the manner required by 21 C.F.R. § 1143.3(b), for example: Eonsmoke Juul Compatible Pods – Menthol, Eonsmoke Juul Compatible Pods – Caffe Latte, Eonsmoke Juul Compatible Pods – Mango, Eonsmoke Salt Nicotine E-Liquids – Donut Cream, Eonsmoke Salt Nicotine E-Liquids – Candy Apple Melon, Eonsmoke Disposable ENDS – Blue Raspberry, Eonsmoke Disposable ENDS – Kiwi Strawberry, and Eonsmoke Disposable ENDS – Mint.
Under 21 C.F.R. § 1143.3(b), advertising for cigarette tobacco, roll-your-own tobacco, and covered tobacco products (other than cigars), such as certain ENDS products, must bear the following warning statement:
WARNING: This product contains nicotine. Nicotine is an addictive chemical.
For cigarette tobacco, roll-your-own tobacco, and covered tobacco products other than cigars, it is unlawful for a tobacco product manufacturer, packager, importer, distributor, or retailer of the tobacco product to advertise or cause to be advertised within the United States any tobacco product unless each advertisement bears the required warning statement (21 C.F.R. § 1143.3(b)). Further, the required warning statement must meet the requirements of 21 C.F.R. § 1143.3(b)(2). Under 21 C.F.R. § 1143.1, a “covered tobacco product” is defined as any tobacco product deemed to be subject to the FD&C Act under 21 C.F.R. § 1100.2, excluding components or parts not made or derived from tobacco. Before 21 C.F.R. § 1100.2 became effective, only cigarettes, smokeless tobacco, roll-your-own tobacco, and cigarette tobacco were subject to chapter IX of the FD&C Act. 21 C.F.R. § 1100.2 deems all other tobacco products, except accessories of such tobacco products, subject to chapter IX and its implementing regulations. The products cited in this violation are “covered tobacco products.” Under section 903(a)(7)(B) of the FD&C Act (21 U.S.C. § 387c(a)(7)(B)), tobacco products are misbranded if sold or distributed in violation of regulations prescribed under section 906(d) of the FD&C Act, including those within 21 C.F.R. Part 1143. Because advertising regarding these ENDS products on social media accounts posted on behalf of, and by, Eonsmoke, LLC and on your website does not include the required nicotine warning statement for these products, in the manner required by 21 C.F.R. § 1143.3(b), the ENDS products are misbranded under section 903(a)(7)(B) of the FD&C Act (21 U.S.C. § 387c(a)(7)(B)).
In addition, a tobacco product is misbranded under section 903(a)(7)(A) of the FD&C Act (21 U.S.C. § 387c(a)(7)(A)) if, in the case of any tobacco product distributed or offered for sale in any State, its advertising is false or misleading in any particular. Under section 201(n) of the FD&C Act (21 U.S.C. § 321(n)), in determining whether labeling and/or advertising is misleading, the agency considers, among other things, the failure to reveal material facts concerning the consequences that may result from the customary or usual use of the product. Because the posts on your twitter account, the verified Twitter account of Scott Disick, and the verified Instagram account of Mia Khalifa mentioned above do not include the required nicotine warning statement, the e-liquid products are misbranded under section 903(a)(7)(A) of the FD&C Act (21 U.S.C. § 387c(a)(7)(A)).
Tobacco Products Without Required Ingredient Listing Submissions Are Misbranded
FDA has determined that you have not provided an ingredient listing to FDA as required by section 904(a)(1). Section 904(a)(1) requires each tobacco product manufacturer or importer, or agents thereof to provide “a listing of ingredients, including tobacco, substances, compounds, and additives that are… added by the manufacturer to the tobacco, paper filter, or other part of each tobacco product by brand and by quantity in each brand and sub-brand.”
Because you have not provided FDA with an ingredient listing, FDA has determined that the products listed below, which Eonsmoke, LLC imports and distributes to customers in the United States, are misbranded under section 903(a)(10)(A) of the FD&C Act (21 U.S.C. § 387d(a)(1) because you failed to comply with requirements prescribed under section 904 of the FD&C Act (21 U.S.C. §387d). The introduction into interstate commerce of any tobacco product that is misbranded is a prohibited act under section 301(a) of the FD&C Act (21 U.S.C. § 331(a)). In addition, the failure to provide any information required by section 904 is a prohibited act under section 301(q)(1)(B) of the FD&C Act (21 U.S.C. § 331(q)(1)(B)).
|
20. Eonsmoke Pods Multipack 6% |
39. 4X Grape 6.5% |
|
21. Eonsmoke Pods Pineapple 4% |
40. 4X Green Apple 6.8% |
|
22. Eonsmoke Pods Pineapple 6% |
41. 4X Kiwi Strawberry 6.8% |
|
23. Eonsmoke Pods Pineapple 7% |
42. 4X Mango Pineapple Guava 6.5% |
|
24. Eonsmoke Pods Pink Lemonade 4% |
43. 4X Multipack 6.5% |
|
25. Eonsmoke Pods Pink Lemonade 6% |
44. 4X Peach Madness 6.8% |
|
26. Eonsmoke Pods Silky Strawberry 4% |
45. 4X Raspberry Mint Lemonade 6.5% |
|
27. Eonsmoke Pods Silky Strawberry 6% |
46. 4X Sour Berry Belts 6.5% |
|
28. Eonsmoke Pods Silky Strawberry 7% |
47. 4X Sour Gummy 6.5% |
|
29. Eonsmoke Pods Tobacco 4% |
48. Eonsmoke Blue Raspberry Disposable 7% |
|
30. Eonsmoke Pods Tobacco pods 6% |
49. Eonsmoke Cubano Disposable 7% |
|
31. Eonsmoke Pods Watermelon 4% |
50. Eonsmoke Fresh Mint Disposable 7% |
|
|
51. Eonsmoke Kiwi Strawberry Disposable 7% |
|
33. Eonsmoke Pods Watermelon 7% |
52. Eonsmoke Lush Ice Disposable 7% |
|
34. Eonsmoke Pods Wrangler 4% |
53. Eonsmoke Mango Disposable 7% |
|
35. Eonsmoke Pods Wrangler 6% |
54. Eonsmoke Peach Rings Disposable 7% |
|
36. Lush ice 6% pods pack |
55. Eonsmoke Sour Gummy Disposable 7% |
|
37. 4X Blue Blackberry 6.5% |
56. Eonsmoke Sweet Grape Disposable 7% |
|
38. 4X Blue Raspberry 6.8% |
|
Conclusion and Requested Actions
The violations discussed in this letter do not necessarily constitute an exhaustive list. You should immediately correct the violations that are referenced above, as well as violations that are the same as or similar to those stated above, and take any necessary actions to bring your tobacco products into compliance with the FD&C Act.
It is your responsibility to ensure that your tobacco products and all related labeling and/or advertising on your website, on any other websites (including e-commerce, social networking, or search engine websites), and in any other media in which you advertise comply with each applicable provision of the FD&C Act and FDA’s implementing regulations. Failure to ensure full compliance with the FD&C Act may result in FDA’s initiating further action, including, but not limited to, civil money penalties, seizure, and/or injunction. Please note that any adulterated and/or misbranded tobacco products offered for import into the United States are subject to detention and refusal of admission.
Please submit a written response to this letter within 15 working days from the date of receipt describing your corrective actions, including the dates on which you discontinued the violative sale, and/or distribution of these tobacco products and your plan for maintaining compliance with the FD&C Act. If you do not believe that your products are in violation of the FD&C Act, include your reasoning and any supporting information for our consideration. You can find the FD&C Act through links on FDA’s homepage at http://www.fda.gov.
Please note your reference number, ER190031, in your response and direct your response to the following address:
DEM-WL Response, Office of Compliance and Enforcement
FDA Center for Tobacco Products
c/o Document Control Center
Building 71, Room G335
10903 New Hampshire Avenue
Silver Spring, MD 20993-0002
If you have any questions about the content of this letter, please contact Lillian Ortega at (240) 402-9041 or Lillian.Ortega@fda.hhs.gov.
Sincerely,
/S/
Ann Simoneau, J.D.
Director
Office of Compliance and Enforcement
Center for Tobacco Products
VIA Electronic Mail
cc:
Eonsmoke, LLC
info@eonsmoke.com
customerservice@eonsmoke.com
wholesale@eonsmoke.com
affiliate@eonsmoke.com
press@eonsmoke.com
international@eonsmoke.com
Michael Tolmach
MichaelTolmach@gmail.com
GoDaddy.com, LLC
abuse@godaddy.com
Hetzner Online GmbH
abuse@hetzner.de