Inspectional Observations and Citations
Observations are listed on an FDA Form 483 in order of risk significance by the investigator. The format of any single observation begins with a statement based in a citation of law, regulation or Act and is followed by a statement of specific conditions observed during the inspection. For a description of the citations and the data in the attached spreadsheets, please see A Short Description of Citations.
The standardized citations on the issued FDA Form 483 provides a detailed description of the investigators observation(s), the short and long plain language citations describing the laws, regulations or Acts and the Code of Federal Regulations (CFR) or other reference guidances. Multiple citations may exist for each CFR reference, addressing specific and distinct elements of the referenced law or Act.
To reduce the complexities of laws and regulations and identify multiple areas for concern, the short description details the specific element which is objectionable for an observation. The long description and reference number provide context for a short description that is cited in an observation.
For examples of inspectional observations and how the standardized citations are used to record observations, please see the ORA Reading Room for examples of recently issued 483s.
A Short Description of Citations
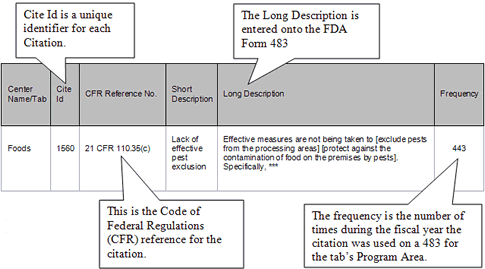
Citations are maintained in a database and are reviewed, edited, and updated on a periodic basis. Citations relate to a Code of Federal Regulations (CFR) reference, and multiple citations may exist for a single CFR reference. To create an FDA Form 483, citations are selected from the pre-established system or database. The long description is entered into the FDA Form 483, ensuring uniformity of presentation, then specific information related to the observation may be entered, and the citations may be ranked by risk significance on the 483. At the present time, the citation database does not contain cites for all areas of the Food Drug and Cosmetic Act or the regulations enforced by FDA, so FDA Form 483’s that reference these are written manually and the citations are not available for reporting from this database.
In generated fiscal year reports, a citation has the following display:
Back to Inspection Observations Main Page