PQ/CMC and IDMP
In 2019, FDA attempted to map PQ/CMC data elements to IDMP standards as relevant and document some details to show the similarities and the differences between the FDA’s PQ/CMC Data Standards effort and the ISO IDMP data standards.
Overall Summary of PQ/CMC and ISO IDMP Data Standards
The FDA PQ/CMC and ISO IDMP efforts are both covering data standards in the general domain of drug products and drug substances and therefore as expected, there is some overlap in the terms and concepts used in these efforts. Although there is some overlap in concepts, fundamentally the use case for each of these efforts is different. Please note that the PQ/CMC data standards project is not FDA’s implementation of ISO IDMP Standards. The FDA, European Medicines Agency and WHO-Uppsala Monitoring Centre established the Global IDMP Working Group (GIDWIG) to conduct projects that would lead to the global implementation of the IDMP standards. Following recommendations from a 2019 IDMP Workshop hosted by the World Health Organization, the GIDWG initiated projects on the creation and maintenance of global identifiers, e.g., substance and dose form, to be used in the generation of global pharmaceutical product IDs (PhPIDs).
- Goals & Objectives
- PQ/CMC – The overall goal of the PQ/CMC project is to support Application review, to pre-process data, to populate review templates, to search for data (e.g., related to FARs, AEs, shortage), for inspections and general data retrieval. The objective of the effort is to develop structured computable data standards and terminologies for the CMC content that comes in Module 2’s Quality Overall Summary, and Module 3 of the ICH eCTD submission. This will only include sections of Module 3 that are amenable to structuring and standardization and will bring value to the FDA CMC review process. See FDA PQ/CMC webpage
- ISO IDMP – IDMP intends to provide the basis, with consistent information and common terminologies, to uniquely identify and describe marketed medicinal products to support global exchange of product information to improve pharmacovigilance and, traceability and supply chain integrity.
- Data Standards for different time point in drug development & approval lifecycle
- PQ/CMC – These data standards are designed for submissions of CMC data in ICH eCTD Module 2 and Module 3 for review and approval of a drug application.
- ISO IDMP – For FDA, IDMP provides the basis, with consistent information and common terminologies, to uniquely identify and describe globally marketed medicinal products to improve pharmacovigilance and, traceability and supply chain integrity. FDA does not support the use of the IDMP standards for use in premarket investigational products.
Besides the different use cases that PQ/CMC and ISO IDMP support, there are some additional differences in the level of granularity to which each of these data standards were developed.
- Level of granularity
- PQ/CMC – Designed and developed to be implemented in support of product assessment and therefore provides detailed data element definitions, supporting controlled terminology (where applicable) and the mandatory/optional aspect for each element to allow for consistent and standardized data in various sections of Module 3 in ICH eCTD.
- ISO IDMP – High level definitions for concepts, but the controlled terminology has not yet been developed. The IDMP working group has not addressed most controlled terminology for the 11238 and 11615 standards. This has been left to be developed by the implementers to make it region specific. Some of the recent efforts at EMA, such as SPOR are beginning to develop controlled terminologies for some parts of IDMP that are in scope of SPOR implementation.
- Data Type/Format
- PQ/CMC – FDA PQ/CMC data elements are bound to simple data types. Example, string, integer, etc. to make them easy to understand and get domain expert’s feedback from the industry. When PQ/CMC data elements are represented in HL7 FHIR, these terms are then leveraging the HL7 FHIR complex data types.
- ISO IDMP – ISO IDMP Standards data models are bound to complex data type standard – ISO 21090. Although, as EMA and other IDMP Implementation-oriented projects move forward they are likely to be leveraging the HL7 FHIR complex data types for exchange of the IDMP information.
PQ/CMC, ISO IDMP and HL7 FHIR
Although the fundamental use case of PQ/CMC and ISO IDMP effort is different, they are both leveraging the HL7 FHIR standards for exchanging data. Some of the data element requirements of both PQ/CMC and ISO IDMP are represented in many of the same FHIR Resources from the Medication Definition Resource category.
Team members representing both efforts are working together through the HL7 Biomedical Research & Regulation (BR&R) Work Group (WG) to align the common/overlapping semantics to ensure consistent use of the FHIR Resources (when applicable).
Why is FDA mapping with IDMP standards?
There are clearly overlaps between PQ/CMC data elements and some of the ISO IDMP Standards. FDA is aligning with the relevant IDMP concepts for the following reasons:
- Commitment to data interoperability between regulators
- Commitment to use of established public standards where relevant Minimizing duplicate data submission, when possible
PQ/CMC and ISO IDMP High-level Mapping/Alignment
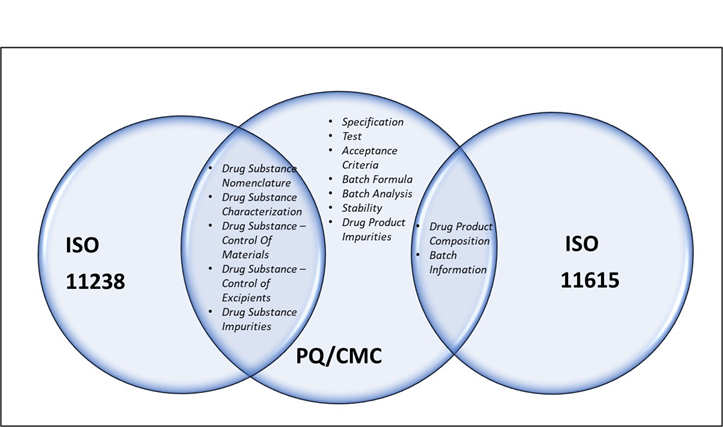
Figure 1 below shows which areas of drug product and drug substance domain are in scope of PQ/CMC and which parts overlap with ISO 11238 and ISO 11615 standards. Please Note: This is a high-level mapping/alignment, and the data element details are not represented here. The sub-domain labels presented in the Venn diagram below are PQ/CMC centric – shows the PQ/CMC categories/sub-domains as published in the two FDA Federal Register Notices (FRNs) in 2017 and 2022. The focus is to show which sub-domains of PQ/CMC Phase 1 have overlapping semantics with ISO 11238 and 11615. It is not intended to show all the areas that are in IDMP scope.
Figure 1: Overlap of concepts between PQ/CMC and ISO IDMP
Table 1: High-level Mapping/Alignment between PQ/CMC and ISO IDMP
|
# |
PQ/CMC Data Element Domains |
ISO 11238 & 11615 Model/Classes [2017] |
Comments |
|---|---|---|---|
|
1 |
Quality Specification |
Not Applicable |
FDA’s review/assessment use case and perspective in CMC is from a point of (Quality) Specification for the various types of materials (product, substance, components, excipients, raw materials, etc.). FDA leverages the core principle of the ICH Q6A definition of a Specification as – “A list of tests, references to analytical procedures, and appropriate acceptance criteria which are numerical limits, ranges, or other criteria for the tests described. It establishes the set of criteria to which a drug substance or drug product should conform to be considered acceptable for its intended use”. This definition is reflected in the following three regulatory definitions of Specifications: 21 CFR 314.3; 514.8 (iv); 600.3 (kk) Although ISO 11238 has identified the same (as above) ICH definition for a Specification, the perspective appears to be from a Substance point of view. Because Product is not a Substance, therefore the ICH definition of Specification may not be completely applicable. This ICH definition of the Specification does not appear to be reflected in the ISO 11238 Specified Substance Group 4 (SSG4) data model. |
|
2 |
Test |
Not Applicable |
|
|
3 |
Acceptance Criteria |
Not Applicable |
|
|
4 |
Batch Information |
ISO 11615: Packaged Medicinal Product, Manufactured Item, … |
Although ISO IDMP 11615 refers to a Batch of a Medicinal Product, it only captures the Batch identifier and Expiration Date of the Packaged Medicinal Product. For CMC review of a Drug Product, the FDA CMC Reviewers need additional information regarding the Batch, including, batch size, manufacturing sites, and testing sites. This information is not captured in the ISO 11615 standard. |
|
5 |
Batch Analysis |
Not Applicable |
PQ/CMC refers to the results associated with analyzing or evaluating the quality of a drug substance or a drug product batch based on the release specification. For CMC review of a Drug Product, the FDA Reviewers utilize these analysis results. This level of detail Batch analysis result was not found in ISO 11615 |
|
6 |
Stability Study |
Not Applicable |
PQ/CMC Stability Study information is intended to provide the structured plan of a formal investigation to continually evaluate the physical, chemical, biological, microbiological, biochemical, and immunochemical attributes of a drug substance or a drug product as a function of time. FDA believes that this information was not planned to be in scope of the either ISO 11238 or ISO11615. |
|
7 |
Nomenclature & Structure of Drug Substance |
ISO 11238: Substance, SubstanceName, SubstanceCode, Structure, StructuralRepresentation |
PQ/CMC data elements provide the description of how a substance is named and graphically rendered. The data elements in this domain have a direct overlap with ISO 11238 standards.
|
|
8 |
Drug Substance Characterization |
ISO 11238: Substance, ReferenceSource, ReferenceSourceDocument |
PQ/CMC data elements identify for the structural and functional characterization of a drug substance using orthogonal analytical techniques. Although there appears to be support for Substance characterization in ISO 11238, it is not clear where the best mapping should be. For Chemical Substance (Reference: Figure 19 of 11238 Standard) FDA’s best guess is that these data elements may map to a combination of Property class as well as the Reference_Source and Reference_Source_Document classes. Since the terminology for the Reference classes has not been defined, it is hard to make this determination. |
|
9 |
Drug Product Composition |
ISO 11615: MedicinalProduct, MedicinalProductName, Ingredient |
PQ/CMC data elements in this domain provide the description of the drug product and the identification & amount of the components in a unit of a drug product. The core concept of drug product aligns with ISO 11615 standards for Medicinal Product. |
|
10 |
Batch Formula |
Not Applicable |
PQ/CMC data elements in this domain provide the description of the drug product and the identification & amount of the components in a unit of a drug product. This is the recipe for making the drug product. For CMC review of a Drug Product, the FDA Reviewers need the batch formula details. This level of detail for a Batch/Lot does not appear to be in scope of ISO IDMP 11615 standard. |
|
11 |
Drug Substance – Control of Materials |
ISO 11238: Manufacturing Material, Source material, Organism, etc. |
PQ/CMC data elements in this domain provide the evidence of the identity, composition and origin of the raw materials and the drug substance. |
|
12 |
Drug Product – Control of Excipients |
ISO 11238: Source material, Organism, etc. |
PQ/CMC data elements in this domain provide the evidence of the identity, composition and origin of the raw materials. As per our understanding of the ISO 11238 standard, an excipient is considered as a Substance.
|
|
13 |
Drug Substance Impurities
|
ISO 11238: Impurity, Structural Representation |
PQ/CMC data elements in this domain provide details for the content in a drug substance that is unintentionally present and is generally found in reasonably low levels. |
|
14 |
Drug Product Impurities
|
|
PQ/CMC data elements in this domain provides details for any component of the drug product which is not the chemical entity defined as the drug substance or an excipient in the drug product. This includes process-related impurities and contaminants, product-related impurities including degradation products. ISO 11615 does not appear to specify or model drug product impurities. This may be out-of-scope for ISO11615. Since some substance impurities may eventually be identified as drug product impurities, for now FDA is considering the mapping of Drug Impurities data elements to IDMP same as the mappings for Drug Substance Impurities. |
Stay Connected
- For additional information/support from PQ/CMC Team, please contact PQ-CMC@fda.hhs.gov.