FY2016 Regulatory Science Report: Nanotechnology: Physiochemical Characterization of Nano-Sized Drug Products
This section contains only new information from FY2016. For background scientific information and outcomes from previous years on this research topic, please refer to the FY15 Regulatory Science Research Report on Nanotechnology: Physiochemical Characterization of Nano-Sized Drug Products.
Introduction
Nano-sized drug products, such as emulsions, liposomes, protein-drug complexes and iron colloids, are complex drug products that require comprehensive physicochemical characterization to gain insight into product quality that can eventually affect in vivo performance. For many generic nano-products, equivalence in physicochemical properties (such as particle morphology, surface potential, size distribution, carrier composition, and state of the encapsulated drug) must be demonstrated. In many instances, characterization of these products is challenging due to the inherent complexity of the formulation. Additionally, the test product should possess a drug release profile that compares to the profile of the reference product, since bioavailability can be markedly influenced by differences in drug release from the formulations. Despite the increasing number of nano-sized products on the market, compendial or bio-relevant in vitro drug release assays for these complex dosage forms are in short supply. This shortage, along with complexities in physicochemical properties, poses a significant challenge to science-based policy development as well as the regulatory review of this product category.
Research
A collaborative research program has been established with the FDA Advanced Characterization Facility (ACF) at the Office of Science and Engineering Laboratories (OSEL), Center for Devices and Radiological Health (CDRH) through which physicochemical characterization of several emulsion and liposome drug products are being conducted. Advanced analytical methods such as cryo-electron microscopy (cryo-EM) enable FDA researchers to image these nano-sized drugs in a frozen hydrated state to better understand their unique structural properties. Collaborative projects in this area advance our understanding of the use of nanotechnology in developing generic drug products.
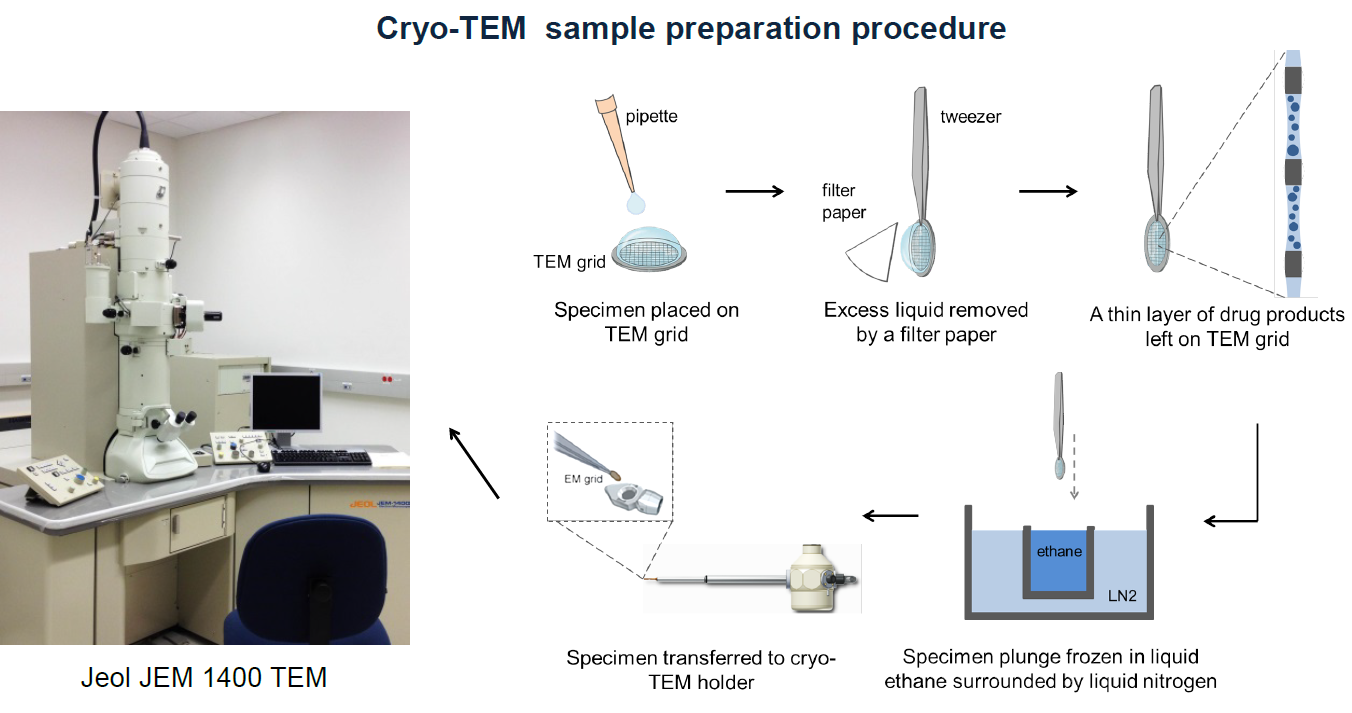
Figure 1a (courtesy of Dr. Yong Wu and Dr. Jiwen Zheng). Procedure for preparing samples for cryo-TEM (transmission electron microscopy). A drop of nano-sized drug is placed on a TEM grid and excess liquid is removed to leave a thin film of the sample in the hole of the grid. The grid is plunged into a bath of liquid ethane, that is about -180°C to rapidly freeze the sample. This frozen sample is then imaged using a transmission electron microscope to provide structural information of the drug products in nanometer-scale resolution.
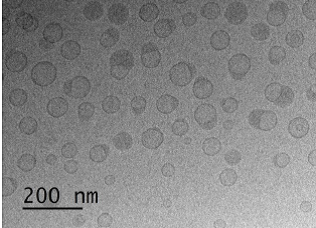
Images generated by cryo-EM provide the FDA with detailed information of the size and structure of drug products. As shown in Figure 2, propofol products can be imaged by cryo-TEM and the different components of the drug formulation can be seen.
Figure 2 (courtesy of Dr. Yong Wu and Dr. Jiwen Zheng). Cryo-TEM images of propofol emulsion illustrating the presence of oil droplets (dark circles), liposomes (ring structures), and emulsion-liposome complexes.
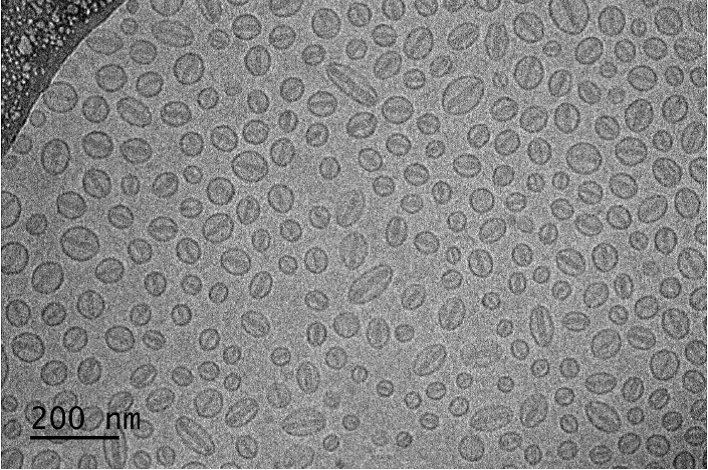
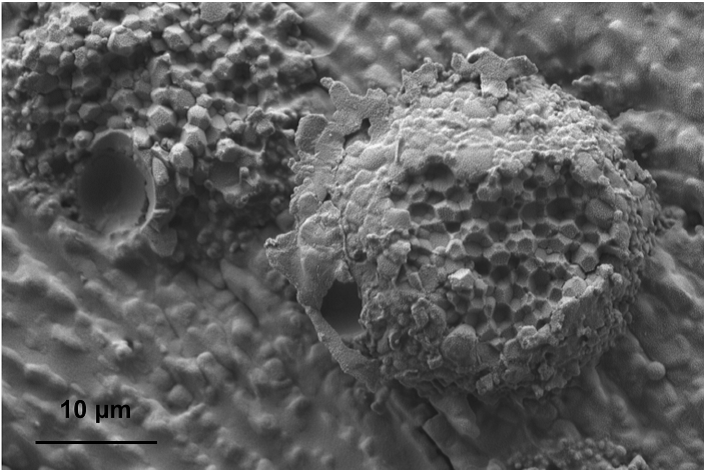
In addition to size analysis, cryo-EM enables analysis of complex structures and morphology within nano-scale drug products. The abundance and homogeneity of these features can be assessed and compared to ensure that they are representative of the product and if they are critical to the formulation’s drug performance. For example, as shown in the image below, some liposomes of Doxil (doxorubicin liposomal) have an elongated “coffee bean” like shape. In addition, the “honeycomb” structure of multi-vesicular liposomal bupivacaine can be observed by using cryo-SEM (scanning electron microscopy) through freeze fracture. This multi-vesicular structure increases the drug release duration, ensuring sustained pain relief.
Figure 3 Cryo-TEM of Doxil (liposomal doxorubicin) showing their “coffee bean” like shape (courtesy of Dr. Yong Wu and Dr. Jiwen Zheng).
Figure 4. Cryo-SEM of multi-vesicular bupivacaine liposome showing its “honeycomb” structure (courtesy of Dr. Yong Wu and Dr. Jiwen Zheng).
In addition to cryo-EM, we test alternative analytical methods to assess drug size, structure, and release with the aim of improving the FDA’s understanding and assessment of potential generic products. For example, we are testing new technologies to measure the concentration of drug particles in nano-size drug products as well as alternative methodologies, proposed by the public, to measure the rapid “burst” release of some products. These new tools and analytical ideas help to ensure that the latest and most accurate methods are used by the FDA.
Figure 5 (Petrochenko, et al., AAPS 2016). Paclitaxel protein-bound suspensions undergo a burst release below a critical concentration. Particles rapidly dissolve releasing drug into solution.
Figure 6 (Petrochenko, et al., AAPS 2016). UV-Vis provides a direct measure of albumin and paclitaxel concentration.
Figure 7 (Petrochenko, et al., AAPS 2016). Turbidity measurements, such as DLS scattering intensity, indicate a change in particle concentration and are an indirect measure of drug release.
ORS staff facilitating research in this area
- Darby Kozak, Yan Wang, Peter Petrochenko and Stephanie Choi
Projects and Collaborators
- Internal Project: Characterization of Nano-Sized Generic Drug Products
- FDA Collaborator: Jiwen Zheng, Yong Wu
- FDA Center/Office/Division: CDRH/OSEL/DBCMS
Publications and Presentations
- Core size determination and structural characterization of intravenous iron complexes by cryogenic transmission electron microscopy. Yong Wu, Peter Petrochenko, Lynn Chen, Sook Yee Wong, Mohammad Absar, Stephanie Choi, Jiwen Zheng, International journal of pharmaceutics 505.1 (2016): 167-174.
- Continuous Monitoring of Albumin-Bound Paclitaxel Dissolution Profiles Using Dynamic Light Scattering and In Situ UV/Vis Fiber-Optic Probes. Peter Petrochenko, Sook Wong, Yong Wu, Jiwen Zheng, Xiaoming Xu, Stephanie Choi, Darby Kozak. AAPS Denver, CO (Nov 13–17, 2016)
- Scientific Considerations for Generic Cyclosporine Ophthalmic Emulsion In Vitro Bioequivalence Studies. Mohammad S. Absar, and Stephanie Choi. AAPS Denver, CO (Nov 13–17, 2016)
Outcomes
- Product-Specific guidance on Difluprednate ophthalmic emulsion (Jan 2016)
- Product-Specific guidance on Propofol injectable emulsion (Jun 2016)
- Revision of Product-Specific guidance on Cyclosporine ophthalmic emulsion (Oct 2016)
- Product-Specific guidance on Iron dextran Injection (Oct 2016